Metabolic dysfunction-associated steatotic disease (MASLD) is the most common cause of chronic liver disease in children and adolescents. The development of MASLD is associated with dietary habits, and dietary intake characteristics are a relevant risk factor. The aim of the present study was to analyze dietary intake characteristics in children and adolescents and study how diet varies in subjects with and without MASLD.
MethodsA cross-sectional study was conducted that included children and adolescents from 8 to 18 years of age. The criteria for MASLD included steatosis on abdominal ultrasound study and meeting at least one metabolic syndrome criterion. Obesity was diagnosed based on the age-appropriate body mass index. Dietary habits were evaluated utilizing a 2-day food register collected on different representative days (Tuesday and Sunday), focusing on dietary energy intake, macronutrients, polyunsaturated fatty acids, trans fats, dietary fiber, and antioxidants.
ResultsA total of 89 children and adolescents were evaluated; 50 (56%) were females and 21 (24%) presented with MASLD. The MASLD group had lower intake of protein (median [interquartile range] 76.6 g [57.7−87.7] vs. 85.8 [71.0−114.1], p = 0.035), vitamin E (mean [standard deviation] 6.0 [2.8] vs. 8.0 [6.2], p = 0.040), zinc (7.9 [2.7] vs. 10.3 [4.9], p = 0.031), and iron (9.9 [2.5] vs. 12.2 [5.4], p = 0.009), compared with the non-MASLD group. After confounding variable adjustment, only the waist-to-height ratio was associated with MASLD.
ConclusionThe quantity and quality of foods may be related to MASLD, and abdominal obesity should be prevented in childhood.
La enfermedad hepática esteatósica asociada a disfunción metabólica (MASLD) es la causa más común de enfermedad hepática crónica en niños y adolescentes. El desarrollo de MASLD está asociado con los hábitos alimentarios y las características de la ingesta dietética son un factor de riesgo relevante. Este estudio tuvo como objetivo analizar las características de la ingesta dietética en niñas, niños y adolescentes e investigar cómo varía la dieta entre aquellos con y sin MASLD.
MétodosEste fue un estudio transversal, que incluyó niñas, niños y adolescentes de 8 a 18 años. Los criterios para MASLD incluyeron esteatosis en la ecografía abdominal y cumplir con al menos un criterio de síndrome metabólico. La obesidad se diagnosticó con base en el índice de masa corporal adecuado para la edad. Los hábitos alimentarios se evaluaron utilizando un registro de alimentos de dos días recopilado en diferentes días representativos (martes y domingo), centrándose en la ingesta dietética de energía, macronutrientes, ácidos grasos poliinsaturados, grasas trans, fibra dietética y antioxidantes.
ResultadosSe evaluaron ochenta y nueve niñas, niños y adolescentes, 50 (56%) de sexo femenino y 21 (24%) con MASLD. El grupo con la enfermedad presentó menor ingesta de proteínas [mediana (rango intercuartil) 76.6 g (57.7−87.7) vs. 85.8 (71.0−114.1), p = 0.035], así como de vitamina E [media (desviación estándar) 6.0 (2.8) vs. 8.0 (6.2), p = 0.040], zinc [7.9 (2.7) vs. 10.3 (4.9), p = 0.031] y hierro [9.9 (2.5) vs. 12.2 (5.4), p = 0.009] en comparación con el grupo sin MASLD. Tras el ajuste por variables de confusión, solo el índice cintura-talla se asoció con MASLD.
ConclusiónLa cantidad y calidad de los alimentos pueden estar relacionadas con MASLD y la obesidad abdominal debe combatirse desde la infancia.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is currently the main cause of chronic liver disease in children and adolescents, with estimated prevalence rates of 9.6% and 38% among children and adolescents with normal weight and obesity, respectively, depending on study location, nutritional status, and diagnostic methods.1,2 In children and adolescents, symptoms may be absent or mild, and so diagnosis is often incidental.1 Nevertheless, MASLD severity can progress, even in childhood, and persist into adulthood. It appears to have a more severe phenotype than in adults, with up to 15% of children with MASLD in stage 3.3
Insulin resistance and obesity are among the most important risk factors for MASLD in children and adolescents. Nevertheless, not all patients with obesity have MASLD, whereas patients without obesity may have this diagnosis, and the quality of diet may be a factor in disease onset, progression, and treatment.3,4 Some studies suggest that a high intake of energy and saturated fat and a low intake of fiber, antioxidant nutrients, and omega-3, combined with a sedentary lifestyle, are key in the early onset of MASLD.3,4 Therefore, the present study aimed to analyze the characteristics of food consumption in children and adolescents and investigate how diet varies between those with and without MASLD.
Materials and methodsStudy design and populationA cross-sectional study was carried out to analyze the characteristics of the food consumption of children and adolescents and investigate how this diet varies between those with and without MASLD, in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The study was developed with patients treated at the General Pediatrics Outpatient Clinic of the University Hospital of the Federal University of Bahia. Patients of both sexes between 8 and 18 years of age were included. Children under 8 years of age were not included, as liver diseases in that age group are more strongly associated with hepatic syndromes and are rarely associated with MASLD. The exclusion criteria were classification as underweight based on the age-appropriate body mass index (BMI); syndromic obesity; pubertal abnormalities; drug use that interferes with hepatic and glycemic metabolism, such as corticosteroids, sulfa drugs, antipsychotics, or amphetamines; and antioxidant supplementation. Patients with hypothyroidism, other liver diseases (viral hepatitis A, B, and C, autoimmune diseases, Wilson’s disease, and hemochromatosis), and systemic and/or acute infectious processes in the last 10 days were also excluded.
Clinical evaluationSociodemographic variables (age, sex, and family income), lifestyle habits (participation in physical activity),5 clinical data, such as pubertal stage6 and the presence of acanthosis nigricans,7 were collected via a structured questionnaire administered by the research team and classified based on the published literature.
MASLD diagnostic criteriaMASLD was diagnosed as the presence of hepatic steatosis on abdominal ultrasonography associated with at least one metabolic syndrome criterion.8 The metabolic syndrome criteria associated with MASLD at the time of diagnosis are BMI ≥ 85th percentile for age/sex (BMI z score ≥ +1) OR waist circumference (WC) > 95th percentile OR the equivalent adjusted for ethnicity; fasting serum glucose ≥ 5.6 mmol/l (≥ 100 mg/dl) OR serum glucose ≥ 11.1 mmol/l (≥ 200 mg/dl) OR 2 -h post-load glucose levels ≥ 7.8 mmol (140 mg/dl) OR HbA1c ≥ 5.7% (39 mmol/l) OR already diagnosed/treated type 2 diabetes OR treatment for type 2 diabetes; blood pressure (BP) < 13 years, BP ≥ 95th percentile OR ≥ 130/80 mmHg (whichever was lower); age ≥ 13 years, 130/85 mmHg OR specific antihypertensive drug treatment; plasma triglyceride age < 10 years, ≥ 1.15 mmol/l (≥ 100 mg/dl); age ≥ 10 years, ≥ 1.70 mmol/l (≥ 150 mg/dl) OR lipid-lowering treatment; and plasma HDL-cholesterol ≤ 1.0 mmol/l (≤ 40 mg/dl) OR lipid-lowering treatment. Abdominal ultrasonography was performed by a single evaluator.
Anthropometric assessmentAnthropometric measurements were standardized and statistically compared. Two evaluators performed the measurements, and the average was employed; the maximum accepted difference was 0.5 cm. Weight was measured via an LD 1050 digital scale®, and height was measured via an LD 1050 stadiometer®, with a scale at 0.1-cm intervals. Anthropometric indicators were assessed on the basis of age- and sex-appropriate BMI9 via the WHO AnthroPlus, 2011 version 16. Patients were divided into 2 groups: normal weight-for-age (−2 < z score < +1) and overweight (z score > +1).9 WC, measured as the minimum circumference between the iliac crest and rib cage,10 was evaluated according to the criteria suggested by Rinella et al.8 Neck circumference (NC) was measured at the midpoint of the neck and evaluated according to Da Silva et al.11 The waist-to-height ratio (WHR) was considered normal when it was ≤ 0.5.12 The conicity index13 ranged from 1.14 to 1.16 for children younger than 9 years of age and from 1.06 to 1.12 for children older than 10 years of age.
Biochemical assessmentBiochemical tests were performed after an 8 -h fast. Alanine aminotransferase (ALT), aspartate transaminase (AST), and gamma glutamyl transpeptidase (GGT) were analyzed via the dry chemistry method. The reference values were those for ALT, which was up to 22 U/l for girls and up to 26 U/l for boys, following the North American Society for Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) (201714 recommendation. AST was considered elevated when it was > 59 U/l, and GGT was considered elevated when it was > 73 U/l, according to laboratory-suggested standards because of the method used. Fasting blood glucose was assessed according to Rinella et al.,8 and serum insulin levels were assessed in accordance with the recommendations of the Brazilian Diabetes Society.15 The homeostatic model assessment of insulin resistance (HOMA-IR) was analyzed based on the value for insulin resistance ≤ 3.0 suggested by Yan et al.16 Total cholesterol and low density lipoprotein cholesterol (LDL-C) were evaluated based on the normal values of the Brazilian Society of Cardiology, 2019−2020.17 High density lipoprotein cholesterol (HDL-C) and triglycerides were evaluated based on the values suggested by Rinella et al.8
Blood levels of vitamin C, E, and A and selenium, zinc, and copper were measured and assessed against normal laboratory ranges: 4.6–15.0 mg/dl, 3–10.0 mg/dl, 0.3−0.7 mg/dl, 20.0–190.0 µg/dl, 70.0–120.0 µg/dl, and 80.0–160.0 µg/dl, respectively.
Dietary assessmentTo understand the population’s dietary intake, a 2-day record was collected for each patient. The dietary records included information, such as meals eaten, mealtimes, preparation, types and quantities of food, and quantities consumed. The dietary records were completed on Tuesday and Sunday. Patients and their guardians received dietary record templates and instructions on completion via an example provided by the team. The 2-day dietary records were completed at home and checked upon their submission. The recording was carried out by the primary caretaker. When needed, further support was provided through serving-size photographs.
The dietary assessment was made utilizing the Dietbox® dietary assessment platform. The intake of energy, carbohydrates, proteins, total lipids, polyunsaturated fatty acids (PUFAs), dietary fiber, and micronutrients was evaluated using the 2002/2005 dietary reference intakes.18 Trans fat intake was determined utilizing the parameters proposed by the World Health Organization in 2023.19
Statistical analysisThe sample consisted of all children and adolescents treated at the aforementioned outpatient clinic. The Statistical Package for the Social Science® version 18.0 program was used for the data analysis. Categorical variables were expressed as absolute and relative frequencies, and quantitative variables were expressed as means and standard deviations or medians and interquartile ranges. The participants were divided into 2 groups: with MASLD and without MASLD. The following statistical tests were used to compare groups: t tests and Mann–Whitney U tests for independent samples, to compare the quantitative variables, and chi-square tests and Fisher’s exact tests, to compare the categorical variables. Statistical significance was set at a p < 0.05.
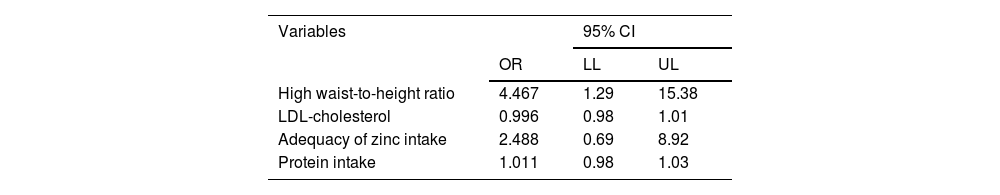
To assess the confounding variables, logistic regression was performed, considering variables with a p value < 0.05 in the bivariate analysis, which did not have collinearity and presented biological plausibility with MASLD: LDL cholesterol, categorized elevated WHR (yes or no), adequate zinc intake (yes or no) and daily protein intake (g/day).
Ethical considerationsThe recommendations of the Declaration of Helsinki, amended by the 64th Annual Assembly of the World Medical Association in 2013, were followed. This study was approved by the Research Ethics Committee of the UFBA School of Nutrition (process number: 1.471.817). Patients and their parents or guardians signed an informed consent form. After the data were collected, all patients had access to outpatient nutrition services. The authors declare that all precautions were taken, preserving patient anonymity.
ResultsAll the children and adolescents treated at the outpatient clinic were invited to participate in the study and there were no refusals or withdrawals, resulting in a total of 89 children and adolescents between 8 and 18 years of age, 50 (56.0%) of whom were females. Of the 89 patients, 21 were diagnosed with MASLD. All had steatosis on abdominal ultrasonography, 20 (95.2%) were overweight or obese, and one (4.8%) had low HDL-C. No patient presented with glycemic changes, diabetes mellitus, changes in blood pressure, or systemic arterial hypertension.
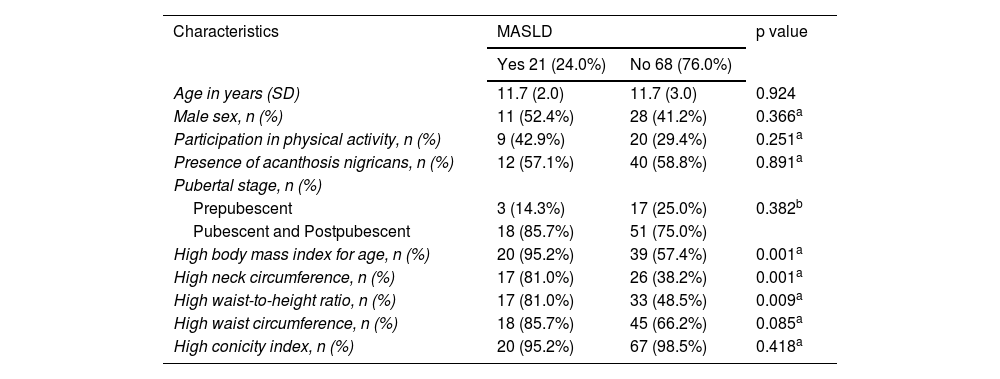
For the comparisons, the participants were divided into 2 groups: those with MASLD and those without MASLD. Sociodemographic characteristics were similar between the 2 groups, and there were significant differences in the anthropometric indicators, including BMI, NC, and WHR (Table 1).
Demographic and anthropometric characteristics of children and adolescents, with and without MASLD, followed at the Pediatric Outpatient Clinic, 2022.
| Characteristics | MASLD | p value | |
|---|---|---|---|
| Yes 21 (24.0%) | No 68 (76.0%) | ||
| Age in years (SD) | 11.7 (2.0) | 11.7 (3.0) | 0.924 |
| Male sex, n (%) | 11 (52.4%) | 28 (41.2%) | 0.366a |
| Participation in physical activity, n (%) | 9 (42.9%) | 20 (29.4%) | 0.251a |
| Presence of acanthosis nigricans, n (%) | 12 (57.1%) | 40 (58.8%) | 0.891a |
| Pubertal stage, n (%) | |||
| Prepubescent | 3 (14.3%) | 17 (25.0%) | 0.382b |
| Pubescent and Postpubescent | 18 (85.7%) | 51 (75.0%) | |
| High body mass index for age, n (%) | 20 (95.2%) | 39 (57.4%) | 0.001a |
| High neck circumference, n (%) | 17 (81.0%) | 26 (38.2%) | 0.001a |
| High waist-to-height ratio, n (%) | 17 (81.0%) | 33 (48.5%) | 0.009a |
| High waist circumference, n (%) | 18 (85.7%) | 45 (66.2%) | 0.085a |
| High conicity index, n (%) | 20 (95.2%) | 67 (98.5%) | 0.418a |
MASLD: metabolic dysfunction-associated steatotic liver disease; SD: standard deviation.
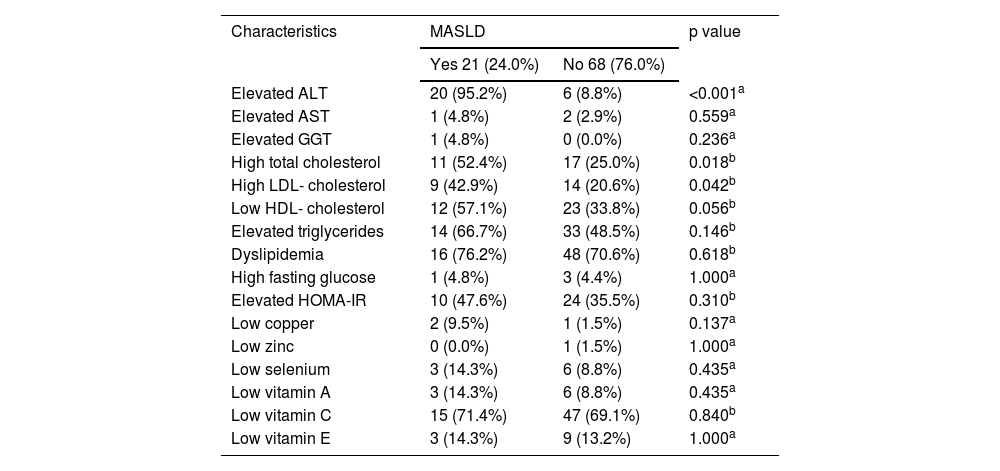
In the MASLD group, the frequencies of high total cholesterol and LDL-C levels were greater. However, when all types of dyslipidemias were analyzed together, there were no differences between groups. The groups differed in terms of ALT adequacy and did not differ with regard to micronutrients or vitamins (Table 2).
Serum levels of liver enzymes, lipid indicators, glucose control, and micronutrients by MASLD status in children and adolescents followed at the Pediatric Outpatient Clinic, 2022.
| Characteristics | MASLD | p value | |
|---|---|---|---|
| Yes 21 (24.0%) | No 68 (76.0%) | ||
| Elevated ALT | 20 (95.2%) | 6 (8.8%) | <0.001a |
| Elevated AST | 1 (4.8%) | 2 (2.9%) | 0.559a |
| Elevated GGT | 1 (4.8%) | 0 (0.0%) | 0.236a |
| High total cholesterol | 11 (52.4%) | 17 (25.0%) | 0.018b |
| High LDL- cholesterol | 9 (42.9%) | 14 (20.6%) | 0.042b |
| Low HDL- cholesterol | 12 (57.1%) | 23 (33.8%) | 0.056b |
| Elevated triglycerides | 14 (66.7%) | 33 (48.5%) | 0.146b |
| Dyslipidemia | 16 (76.2%) | 48 (70.6%) | 0.618b |
| High fasting glucose | 1 (4.8%) | 3 (4.4%) | 1.000a |
| Elevated HOMA-IR | 10 (47.6%) | 24 (35.5%) | 0.310b |
| Low copper | 2 (9.5%) | 1 (1.5%) | 0.137a |
| Low zinc | 0 (0.0%) | 1 (1.5%) | 1.000a |
| Low selenium | 3 (14.3%) | 6 (8.8%) | 0.435a |
| Low vitamin A | 3 (14.3%) | 6 (8.8%) | 0.435a |
| Low vitamin C | 15 (71.4%) | 47 (69.1%) | 0.840b |
| Low vitamin E | 3 (14.3%) | 9 (13.2%) | 1.000a |
Data are expressed as n (%).
ALT: alanine aminotransferase; AST: aspartate transaminase; GGT: gamma glutamyl transpeptidase; LDL-cholesterol: low-density lipoprotein cholesterol; HDL-cholesterol: high-density lipoprotein cholesterol; HOMA-IR: homeostatic model assessment of insulin resistance; MASLD: metabolic dysfunction-associated steatotic liver disease.
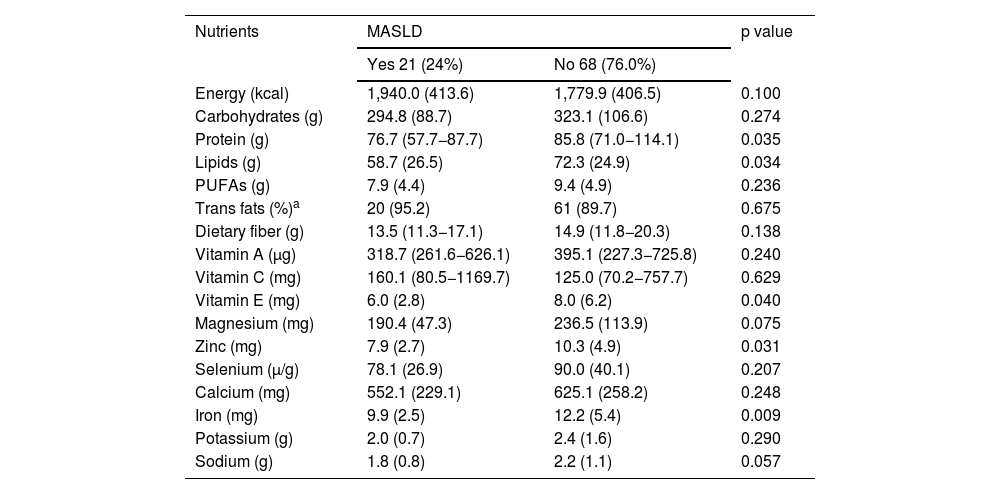
The consumption of trans fat was also evaluated according to adequacy, with a maximum consumption parameter of 1% of total energy intake,19 and consumption was higher than that recommended in both groups (95.2% in the group with MASLD and 89.7% in the group without the disease, p = 0.675 (Table 3).
Comparison of the average daily intake of macronutrients and micronutrients in children and adolescents, with and without MASLD, followed at the Pediatric Outpatient Clinic, 2022.
| Nutrients | MASLD | p value | |
|---|---|---|---|
| Yes 21 (24%) | No 68 (76.0%) | ||
| Energy (kcal) | 1,940.0 (413.6) | 1,779.9 (406.5) | 0.100 |
| Carbohydrates (g) | 294.8 (88.7) | 323.1 (106.6) | 0.274 |
| Protein (g) | 76.7 (57.7−87.7) | 85.8 (71.0−114.1) | 0.035 |
| Lipids (g) | 58.7 (26.5) | 72.3 (24.9) | 0.034 |
| PUFAs (g) | 7.9 (4.4) | 9.4 (4.9) | 0.236 |
| Trans fats (%)a | 20 (95.2) | 61 (89.7) | 0.675 |
| Dietary fiber (g) | 13.5 (11.3−17.1) | 14.9 (11.8−20.3) | 0.138 |
| Vitamin A (µg) | 318.7 (261.6−626.1) | 395.1 (227.3−725.8) | 0.240 |
| Vitamin C (mg) | 160.1 (80.5−1169.7) | 125.0 (70.2−757.7) | 0.629 |
| Vitamin E (mg) | 6.0 (2.8) | 8.0 (6.2) | 0.040 |
| Magnesium (mg) | 190.4 (47.3) | 236.5 (113.9) | 0.075 |
| Zinc (mg) | 7.9 (2.7) | 10.3 (4.9) | 0.031 |
| Selenium (µ/g) | 78.1 (26.9) | 90.0 (40.1) | 0.207 |
| Calcium (mg) | 552.1 (229.1) | 625.1 (258.2) | 0.248 |
| Iron (mg) | 9.9 (2.5) | 12.2 (5.4) | 0.009 |
| Potassium (g) | 2.0 (0.7) | 2.4 (1.6) | 0.290 |
| Sodium (g) | 1.8 (0.8) | 2.2 (1.1) | 0.057 |
Data are expressed as mean (standard deviation) or mean (interquartile range).
MASLD: metabolic dysfunction-associated steatotic liver disease; PUFAs:
polyunsaturated fatty acids.
After adjusting for confounding variables (categorized as elevated WHR [yes or no], LDL-C, adequate zinc intake [yes or no] and daily protein intake [g/day]), MASLD was found to be associated only with WHR (Table 4).
Logistic regression analysis for clinical, anthropometric, and food intake variables of patients with MASLD, followed at the Pediatric Outpatient Clinic, 2022.
| Variables | 95% CI | ||
|---|---|---|---|
| OR | LL | UL | |
| High waist-to-height ratio | 4.467 | 1.29 | 15.38 |
| LDL-cholesterol | 0.996 | 0.98 | 1.01 |
| Adequacy of zinc intake | 2.488 | 0.69 | 8.92 |
| Protein intake | 1.011 | 0.98 | 1.03 |
LDL: low density lipoprotein; CI: confidence interval; LL: lower limit; MASLD: metabolic dysfunction-associated steatotic liver disease; OR: odds ratio; UL: upper limit.
The results of the present study revealed that children and adolescents with MASLD had higher BMIs for age, NC, and waist-to-height ratios, compared with those without MASLD. Our results also showed that MASLD patients consumed lower amounts of protein, vitamin E, zinc, and iron.
There is interest in studying MASLD in children and adolescents because of the worldwide increase in childhood obesity. The frequency of MASLD can be 10–20 times higher in patients with obesity.20 However, central obesity has been shown to be more relevant for MASLD than total body fat.20 In this study, we found that MASLD was more common in patients with a high BMI, WHR, and NC and that the presence of increased total and central body fat was associated with disease.
Some studies have investigated the association between central adiposity and MASLD, and the explanation for this association seems to be related to the fact that visceral adipocytes store and mobilize triglycerides more quickly than adipocytes in other regions, which increases the supply of free fatty acids. In the portal system, this stimulates gluconeogenesis and inhibits hepatic insulin clearance, initiating MASLD development.21,22
With the emergence of MASLD in increasingly younger individuals, dietary intake is another aspect that has recently been shown to be relevant in understanding the development of this disease. Studies have demonstrated that a diet high in calories, simple carbohydrates, and saturated and trans fats, and low in PUFAs, fiber, and antioxidants could be a possible factor triggering disease.4,23
Previous studies have shown that increased protein and low carbohydrate intake reduce fat deposition and plasma cholesterol levels, supporting the prevention of hepatic steatosis.3 In our study, participants with MASLD, on average, had lower protein intake.
Another risk factor associated with MASLD is the intake of poor-quality lipids. Imbalances in the quality and quantity of lipid intake can trigger disease because a high intake of saturated and trans fats promotes liver damage.2,3 However, adequate PUFA intake can prevent the onset of disease by increasing the oxidation of fatty acids and decreasing insulin resistance.2,4 Total dietary lipid intake was lower in the MASLD group. This result can be better evaluated when considering the lower intake of PUFAs and higher intake of trans fat in the MASLD group, which corroborates previous findings and highlights the need for a better-quality diet.
Micronutrients, such as vitamins A, C, and E and the magnesium, copper, zinc and selenium minerals, play nonenzymatic antioxidant roles and can help prevent oxidative stress and lipid peroxidation, which are factors that contribute to the development and progression of MASLD.2,20 The MASLD group tended to have a lower intake of vitamins and minerals linked to oxidative stress prevention. Zinc can be an efficient antagonist of free radical formation; zinc supplementation for 6 months in a previous study was associated with a decrease in malondialdehyde plasma levels.24 Vitamin E has anti-inflammatory and antioxidant properties and stimulates the activity of antioxidant enzymes, including glutathione peroxidase.3
Iron is another nutrient that has been studied in relation to MASLD. That association was not observed in the present study. However, individuals with MASLD tended to have a lower daily intake of iron. There is interest in that nutrient because iron deficiency can be associated with inflammation and low mitochondrial function, conditions present in MASLD.25
High sodium and low potassium intake are linked to metabolic syndrome, and consequently, are risk factors for MASLD, considering the hepatic expression of the syndrome.3 In the present study, there was no difference in the intake of those nutrients between the 2 groups. However, sodium intake exceeded the recommended level, and potassium intake did not meet the recommended level in either group, indicating a risk factor for MASLD. Those results may be due to the high consumption of processed foods, which were not quantitatively evaluated in this study but were observed in the dietary records.
Study limitations include the absence of a processed food intake analysis and the lack of options for investigating the intake of some of the nutrients in the dietary assessment program used. Study strengths include the focus on children and adolescents and the inclusion of individuals with or without overweight or obesity.
Given the significant and gradual increase in obesity and MASLD worldwide, and because young people are exposed to the disease for longer periods when not diagnosed early, we believe that these results reinforce the need to improve nutritional intake in children and adolescents, to prevent obesity and the accumulation of fat in the central region of the body.
CRediT authorship contribution statementPatricia Santos: study conception and design, data acquisition, data analysis and interpretation; writing of the article or critical revision for important intellectual content; final approval of the version to be submitted; Helma Cotrim: study conception and design; critical revision for important intellectual content; final approval of the version to be submitted; Raquel Rocha: study conception and design; critical revision for important intellectual content; final approval of the version to be submitted; Carla Daltro: study conception and design; data analysis and interpretation; critical revision for important intellectual content; final approval of the version to be submitted; Sandra Andrade: data acquisition; Allana Miranda: data acquisition; final approval of the version to be submitted; Allana Castelo: data acquisition; final approval of the version to be submitted.
Financial disclosureThis project was funded by the National Council for Scientific and Technological Development (CNPq) and by the Permanent Program/PROAE-UFBA in the form of research initiation grants. Support from the CNPq, process number 447322/2014-1, was used for biochemical tests, and the company did not participate in this or any other decision. PROAE support was directed to undergraduate scholars (LAS, n. 20557) (KABT, n.18635) and undergraduate students (ICB, n. 26658). CNPq had no role in the design, analysis, or writing of this article. There was no financial support from the authors to finalize or translate the work. We thank the funding companies for their support and encouragement.
Data availability statementThe data are private because they belong to humans. However, if needed, databases can be made available in the future.
The authors declare there are no conflicts of interest.
We would like to thank Luanny Alves de Souza, Luana Milen Varjão, Isadora Cardim Barreto, and Carolina Assumpção Sacramento for performing the data collection.
See related content at DOI: 10.1016/j.rgmxen.2025.06.001, Vázquez-Frias R. Diet is a key factor in the pathogenesis of metabolic dysfunction-associated steatotic liver disease in the pediatric population. Rev Gastroenterol Mex. 2025;90:347-348.