Portal hypertension is the main consequence of cirrhosis and the cause of most of its complications, such as ascites, variceal bleeding, and hepatic encephalopathy. The development of those complications marks the transition from compensated cirrhosis to decompensated cirrhosis, and the latter is associated with poor prognosis. Approximately 50% of cirrhotic patients have gastroesophageal varices. Acute variceal bleeding (AVB) is a medical emergency with high mortality rates ranging from 15 to 25% within 6 weeks. AVB management has evolved in recent years due to new evidence on fluid resuscitation and transfusion support, advances in endoscopic techniques, esophageal stent use, and transjugular intrahepatic portosystemic shunt (TIPS) placement. This consensus aimed to establish recommendations based on the best available evidence and expert opinion from Mexican specialists in gastroenterology for the diagnosis, management, and treatment of AVB in patients with portal hypertension. The goal was to improve clinical decision-making, reduce the associated mortality, and standardize care protocols across the different levels of medical care in Mexico.
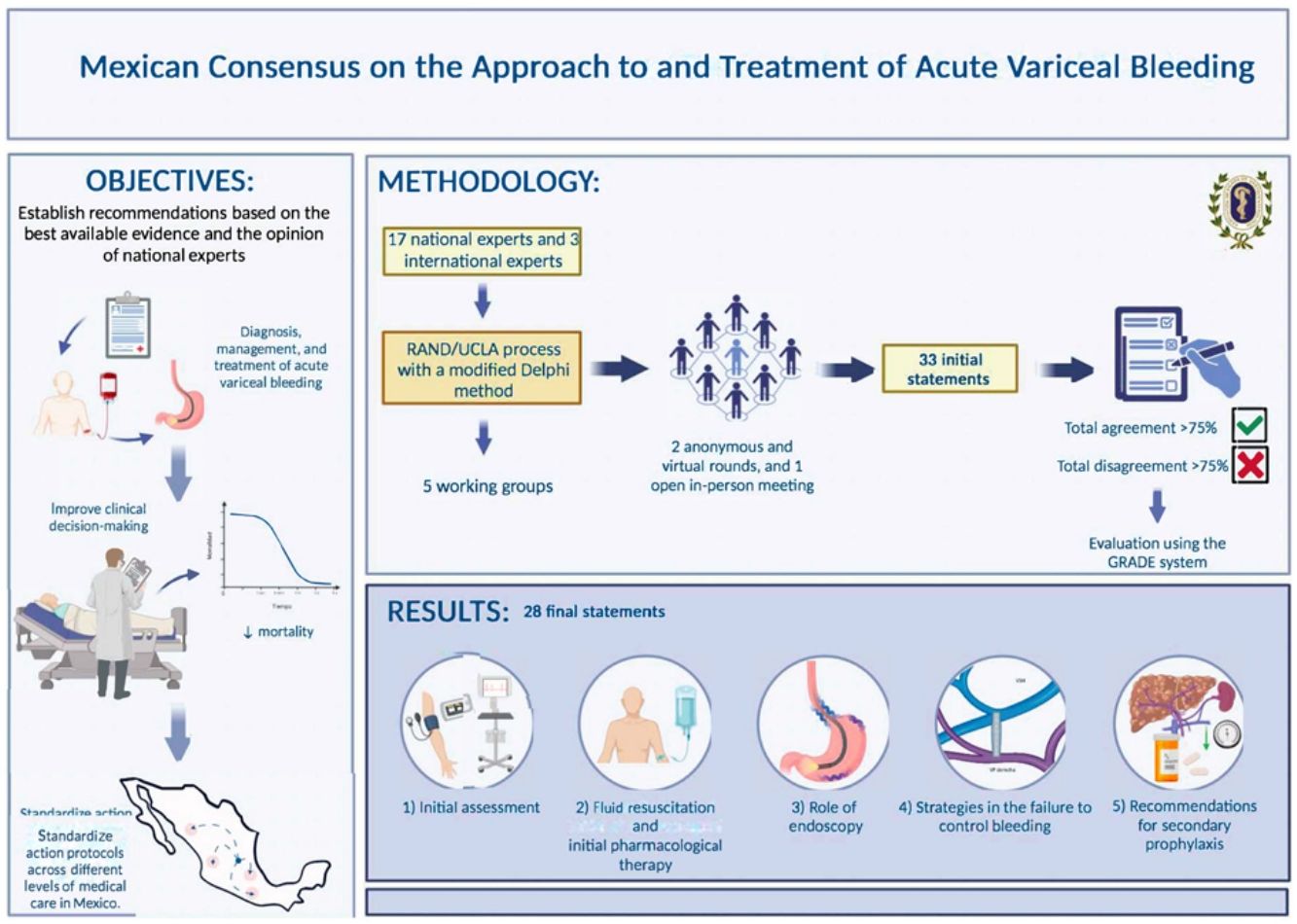
Material and methodsSeventeen national and 3 international experts were divided into five working groups to address five thematic areas: 1) initial evaluation, 2) fluid resuscitation and initial pharmacologic therapy, 3) the role of endoscopy, 4) strategies for managing failed bleeding control, and 5) secondary prophylaxis recommendations. The consensus was developed utilizing the RAND/UCLA process, with a modified Delphi method.
ResultsA total of 28 statements were produced, with specific recommendations on initial fluid resuscitation and transfusion strategy, and highlighting the importance of vasopressor use, the role of endoscopy, and AVB prophylaxis.
ConclusionsThis first Mexican Consensus on Acute Variceal Bleeding establishes practical recommendations for standardizing AVB management in patients with cirrhosis in Mexico, from the initial evaluation to secondary prophylaxis, emphasizing the importance of specific strategies and laying the groundwork for future research.
La hipertensión portal (HTP) es la principal consecuencia de la cirrosis y la causa de la mayoría de sus complicaciones como: ascitis, hemorragia variceal y encefalopatía hepática (EH). El desarrollo de estas complicaciones define la transición de cirrosis compensada a descompensada, esta última con un pobre pronóstico. Alrededor del 50% de los pacientes con cirrosis tienen várices gastroesofágicas. La hemorragia variceal aguda (AVB) es una emergencia que conlleva una elevada mortalidad, siendo del 15% al 25% a las 6 semanas. El manejo de la AVB ha cambiado en los últimos años con la nueva evidencia sobre la reanimación hídrica y soporte transfusional, la implementación de nuevas técnicas de endoscopia, el uso de prótesis esofágicas y la colocación de derivación portosistémica intrahepática transyugular (TIPS).
ObjetivoEl objetivo de este consenso fue establecer recomendaciones basadas en la mejor evidencia disponible y en la opinión de expertos nacionales en gastroenterología para el diagnóstico, manejo y tratamiento de la AVB en pacientes con HTP, con el fin de mejorar la toma de decisiones clínicas, reducir la mortalidad asociada, y estandarizar los protocolos de actuación en los distintos niveles de atención médica en México.
Material y métodosPara lo anterior, se integraron 5 mesas de trabajo con participación de 17 expertos nacionales y 3 internacionales, se abordaron cinco ejes temáticos: 1) evaluación inicial, 2) resucitación y terapia farmacológica inicial, 3) papel de la endoscopia, 4) estrategias en la falla de control de la hemorragia y 5) recomendaciones de profilaxis secundaria. El consenso fue elaborado mediante el proceso RAND/UCLA con el método Delphi modificado.
ResultadosEl resultado de este documento generó un total de 28 enunciados. Se generan recomendaciones especificas sobre la reanimación hidríca inicial y la estrategia de transfusiones. Destaca la importancia sobre uso de vasopresores, papel de la endoscopia y la profilaxis de la AVB.
ConclusionesEl Primer Consenso Mexicano sobre Hemorragia Variceal Aguda establece recomendaciones prácticas para estandarizar su manejo en pacientes con cirrosis en México, desde la evaluación inicial hasta la profilaxis secundaria, destacando la importancia de estrategias específicas y sentando bases para futuras investigaciones.
Liver cirrhosis is a chronic condition with a high mortality rate and is the 14th cause of death in adults worldwide.1 It is classified into 2 prognostic stages: compensated cirrhosis (CC) and decompensated cirrhosis (DC). Portal hypertension (PHT) is the main consequence of liver cirrhosis and the cause of most of its complications, such as ascites, variceal bleeding, and hepatic encephalopathy (HE).2 The development of those complications marks the transition from CC to DC,3 with an important impact on prognosis, given that the mean survival time varies from 12 years in patients with CC to fewer than 2 years, when presenting an episode of clinical decompensation.4
Included in CC is compensated advanced chronic liver disease (cACLD), defined as a liver stiffness measurement (LSM) > 15 kPa in vibration-controlled transient elastography (VCTE) (FibroScan®; Echosens, Paris, France). Two distinct prognostic stages can be identified, according to the presence or absence of clinically significant portal hypertension (CSPHT), which can be determined noninvasively through the LSM and predict the risk of developing clinical decompensation.5–9
Clinical decompensation, defined as the development of any complication derived from PHT (ascites, variceal bleeding, HE, or jaundice), is the most important variable in the prognostic stratification of liver cirrhosis. However, the universally accepted classification of the 2 clinical stages (CC and DC) can oversimplify the course of the liver disease, within which there are different subgroups with different prognoses, depending on the type and number of decompensations.10
The first attempt to characterize the course of clinical decompensation was carried out by D’Amico et al., describing 5 different clinical stages. The first 2 defined CC as stage 1 (without esophageal varices) and stage 2 (with esophageal varices), and the rest were defined according to the type of decompensation: stage 3 (decompensation with acute variceal bleeding [AVB] only), stage 4 (any non-bleeding decompensation), and stage 5 (at least 2 decompensations), with a 5-year mortality rate of 20, 30, and 88%, respectively.4 Afterwards, a later decompensation stage was proposed that is now known as further decompensation, which is defined in Table 1. This progression of the disease occurs in 60% of patients and significantly increases mortality.11,12
Key definitions in the context of variceal bleeding.
| Acute bleeding episode | First 5 days of variceal bleeding |
|---|---|
| Refractory variceal bleeding | Failure to control bleeding or failure in preventing rebleeding |
| Failure to control bleeding or persistent bleeding | Death or the need to modify treatment upon the presentation of any of the following: 1) fresh hematemesis in ≤ 2 h after start of treatment or endoscopic therapy or in patients with nasogastric tube, aspiration of > 100 ml of fresh blood; 2) decrease in hemoglobin ≥ 3 g or in hematocrit of 9% within 24 h; or 3) the development of hypovolemic shock, considering the first 5 days as the episode of acute bleeding |
| Failure to prevent rebleeding or recurrent variceal bleeding | Any clinically significant rebleeding episode (secondary to portal hypertension) after day 5 |
| Clinically significant bleeding | Presence of recurrent melena or hematemesis resulting in hospitalization, blood transfusion, decrease of 3 g in hemoglobin, or death, in the following 6 weeks |
| Beta-blocker responder | Decrease in the HVPG of ≥ 20% of the baseline value or decrease to < 12 mmHg |
| Compensated advanced chronic liver disease (cACLD) | TE with LSM > 15 kPa |
| Clinically significant portal hypertension | HVPG ≥ 10 mmHg or TE with LSM ≥ 25 kPa in patients with cACLD due to viral hepatitis, MASLD without obesity (BMI < 30 kg/m2) and ALD |
ALD: alcohol-associated liver disease; BMI: body mass index; cACLD: compensated advanced chronic liver disease; HVPG: hepatic venous pressure gradient; LSM: liver stiffness measurement; MASLD: metabolic dysfunction-associated steatotic liver disease; TE: transient elastography.
Decompensation in cirrhosis has been proposed to occur in 2 forms: nonacute decompensation (NAD) and acute decompensation (AD). NAD is the most frequent initial form of decompensation and is characterized by the development of grade 2 ascites and/or grade 1 or 2 HE that does not require hospitalization. AD is defined as the acute development of gastrointestinal bleeding, grade 3 or 4 HE, grade 3 ascites and/or complicated ascites (with spontaneous bacterial peritonitis and/or acute kidney injury [AKI]) that requires hospitalization.13
The results of 2 prospective studies (CANONIC and PREDICT), have retrospectively classified patients with AD into 6 subgroups: stable decompensated cirrhosis (SDC), unstable decompensated cirrhosis (UDC), pre-acute-on-chronic liver failure (ACLF) in the following 3 months, and the 3 grades of ACLF.14–16
With the advent of new pharmacologic therapies targeted at reducing fibrosis, the concept of recompensation has been introduced. It describes patients that present with removal, suppression, or cure of the primary etiology of liver cirrhosis, resolution of ascites (with no need for diuretics) and HE (with no need for treatment with lactulose/rifaximin), absence of recurrent variceal bleeding for at least 12 months, and stable improvement in liver function tests (albumin, international normalized ratio [INR], bilirubin).11
Approximately 50% of patients with liver cirrhosis have gastroesophageal varices.17 In patients with CC, the prevalence of gastroesophageal varices ranges from 30 to 40%, with an annual development rate of 7 to 8%,18,19 whereas in patients with DC (Child-Pugh B or C), the prevalence of varices increases from 71.9 to 85%.19
Variceal bleeding is the second most frequent clinical decompensation. It is an emergency that, despite the advances in medical treatment of the past decade, leads to an important increase in mortality, from 15 to 25% at 6 weeks.17 Some risk factors associated with the risk of bleeding are the size of the varices, the presence of cherry-red spots, and the stage of liver disease (Child-Pugh B or C).20
The management of AVB has changed in recent years, as a result of new evidence on fluid resuscitation and transfusion support, the implementation of new endoscopic techniques, the use of esophageal stents, and the placement of transjugular intrahepatic portosystemic shunts (TIPSs).11,21,22Table 1 describes the key definitions of PHT.
The present consensus aimed to establish recommendations based on the best available evidence and expert opinion of national gastroenterology specialists for the diagnosis, management, and treatment of variceal bleeding in patients with PHT, to improve clinical decision-making, reduce the associated mortality, and standardize the care protocols across the different levels of medical care in Mexico. This aim focuses on the implementation of practical guidelines, adapted to the specific needs of the Mexican healthcare system.
MethodologyAt the behest of the Board of Directors and Scientific Committee of the Asociación Mexicana de Gastroenterología A.C. (AMG), 3 general coordinators were designated: Alejandra Noble Lugo (ANL), José Antonio Velarde Ruiz Velasco (JAVRV), and Fátima Higuera de la Tijera (FHT), who equally contributed to the conception of the present manuscript. Five working groups were incorporated, with the participation of 17 national and 3 international experts. The consensus was developed utilizing the RAND/UCLA23 process and a modified Delphi method, carrying out the following phases:
- 1.
Formulation of the problem list: Made by the general coordinators and working group coordinators based on a list of problems to resolve, utilizing patient or problem, intervention, comparison, and outcome (PICO) questions.
- 2.
Review of the scientific evidence: The general coordinators and working group coordinators carried out the search, article classification, and electronic bibliography creation. Each working group then organized the members to start the summarizing of the existing scientific evidence, after which they made the proposed recommendations, based on the PICO questions. All members of the panel had access to the electronic bibliography. The general coordinators reviewed the bibliography, utilizing as the search criteria the words “gastrointestinal bleeding” combined with the following terms: “variceal”, “evaluation”, “cirrhosis”, “acute”, “approach”, “treatment”, “vasopressors”, “ligature”, “mortality”, “esophageal varices”, “gastric varices”, and their Spanish equivalents. The search was conducted on PubMed®, Google Scholar®, Scopus®, Medline®, Embase®, Science Direct®, and the TRIP Database®, for articles published within the time frame of January 2010 and June 2024 and included all publications in English and Spanish. Preference was given to consensuses, guidelines, systematic reviews and meta-analyses, clinical trials, and cohort studies, but was not limited to them. Complementary electronic and manual searches were also conducted in the archives of the Revista de Gastroenterología de México and in all publications up to June 2024 that the coordinators considered relevant.
- 3.
Integration of the panelists: The panelists were selected based on the criteria of clinical experience, recognized prestige in the scientific community, and absence of conflict of interest with the consensus theme. Five working groups, each with a coordinator, were formed, on the following defined thematic areas:
Working group 1: Initial evaluation
Working group 2: Fluid resuscitation and initial pharmacologic therapy
Working group 3: The role of endoscopy
Working group 4: Strategies for managing failed bleeding control
Working group 5: Secondary prophylaxis recommendations
- 4.
Delphi method: Two anonymous, electronic voting rounds and one face-to-face round were carried out. The coordinators formulated 33 statements that were voted on electronically and anonymously, to evaluate writing and content. The consensus participants voted according to the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement. After the first voting round, the coordinators made the corresponding modifications. The statements with complete agreement > 75% were kept and those with complete disagreement > 75% were eliminated. The statements with complete agreement ≤ 75% and complete disagreement ≤ 75% were reviewed and re-structured, after which a total of 31 statements underwent a second electronic and anonymous voting round. According to the comments made, the results of this second vote were submitted to a third in-person vote.
- 5.
International expert feedback: The document was then sent to the feedback committee of experts who evaluated the consensus for its validation.
- 6.
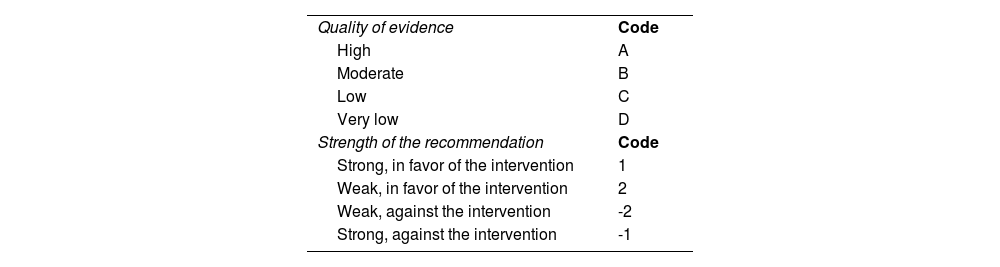
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system evaluation and evidence incorporation: The following definitions were taken into account in the GRADE evaluation of the statements:
- -
Quality of evidence: indicates the extent to which one can be confident that an estimate of effect is accurate and relevant for making recommendations.
- -
Strength of the recommendation: indicates the extent to which one can be confident that the desirable effects of an intervention outweigh its undesirable effects.
The evaluation was carried out by the coordinators of each working group and reviewed by the general coordinators (Table 2).24
Evaluation through the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system and integration of evidence.
| Quality of evidence | Code |
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of the recommendation | Code |
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | -2 |
| Strong, against the intervention | -1 |
According to the Delphi method, the expert panel formulated a total of 28 statements that are presented according to each thematic area, below:
Working group 1. Initial evaluationStatement 1: In patients suspected of having advanced liver disease who present with a first episode of upper gastrointestinal bleeding, the recommendation is to carry out a clinical history and physical examination directed at the search for clinical, biochemical, and paraclinical data of PHT, without delaying the start of medical and pharmacologic management or endoscopic study
In complete agreement: 100%
Quality of evidence: B; Strength of the recommendation: 1
In all patients with gastrointestinal bleeding, a complete clinical history should be carried out that focuses on identifying the risk factors for chronic liver disease, and in patients with known liver cirrhosis, questions directed at the history of clinical decompensations should be asked, to determine the origin of the bleeding (variceal or nonvariceal). PHT is the main consequence of liver cirrhosis and the cause of most of its complications, including variceal bleeding. Between 30 and 40% of patients with CC and up to 85% with DC are estimated to have esophageal varices.18,19,22
Along with the clinical history, suspicion should be based on the clinical data of PHT, such as the presence of splenomegaly (spleen length ≥ 13 cm), collateral circulation, ascites, facial telangiectasias, and lower limb edema, among others. The gold standard for evaluating portal pressure is measuring the hepatic venous pressure gradient (HVPG) that represents the gradient between the hepatic sinusoidal capillary network and the free suprahepatic vein pressure.3 When the HVPG is ≥ 10 mmHg, there is an increased risk of developing esophageal varices, clinical decompensation (ascites, variceal bleeding, or HE), and hepatocellular carcinoma, which is why it is considered CSPHT.5–8 However, it is an expensive and not widely available invasive technique,3,25 making its performance in daily clinical practice inefficient, which has led to the implementation of noninvasive methods for determining the presence of CSPHT. Transient elastography (TE) or VCTE (FibroScan®; Echosens, Paris, France) is a noninvasive technique that measures tissue stiffness.26 LSM through TE has been shown to be useful for diagnosing CSPHT, with a value > 25 kPa predicting CSPHT in 90% of patients.27 Values between 20 and 25 kPa, together with a platelet count < 150 × 109/l or a LSM between 15 and 20 kPa and platelet count < 110 × 109/l, determine at least a 60% risk of CSPHT in most etiologies of cirrhosis.28
PHT leads to the development of passive splenic congestion, which, when added to the increase in arterial inflow as a consequence of splanchnic vasodilation, hyperactivation of the splenic lymphoid tissue, fibrogenesis, and angiogenesis, results in increased stiffness of the spleen.29 Splenic stiffness measurement (SSM) reflects both the fixed component and the dynamic component of PHT,3 which unlike LSM, is not affected by liver disease etiology, liver congestion, inflammation, infiltration, or cholestasis.29
The SSM has been shown to be a good predictor of the presence of esophageal varices, reporting positive predictive values of 91 and 93.4%, with cutoff points > 40.8 kPa and 46.4 kPa, respectively.30 The NICER model, which includes the SSM and LSM, platelet count, and body mass index (BMI), has been shown to be superior to the ANTICIPATE ± NASH model for predicting CSPHT in patients with cACLD of different etiologies (AUC 0.906 [0.864-0.946] vs 0.863 [0.810-0.916]; p = 0.012). Those findings support implementing the SSM in clinical practice.31
The imaging studies of B-mode ultrasound and pulsed wave Doppler ultrasound can reveal data suggestive of CSPHT, such as splenomegaly, umbilical vein recanalization, portosystemic collaterals, and reduced portal vein velocity (< 12 cm/s),29,32 as well as the presence of portal vein thrombosis (PVT), which is a frequent event in patients with liver cirrhosis, with a prevalence of up to 13.92%.33 Patients with PVT have a higher prevalence of variceal bleeding, compared with patients without thrombosis (47.33 vs 19.63%; p < 0.001), regardless of whether the thrombosis is acute or chronic (49.35 vs 43.82%; p = 0.43).34 PVT has also been shown to be associated with an increased risk of death (OR 1.12, p < 0.001), AKI (OR 1.75, p < 0.001), and hepatorenal syndrome (OR 1.62, p < 0.001).35 Therefore, the recommendation is to perform color Doppler ultrasound in all patients that present with acute variceal gastrointestinal bleeding, once hemodynamic stability and bleeding remission are achieved. The abovementioned determinations should not delay the start of pharmacologic medical management or the performance of diagnostic/therapeutic endoscopy.36
Statement 2: In the initial management of the patient with cirrhosis and suspected variceal gastrointestinal bleeding, the recommendation is to evaluate hemodynamic and neurologic status, assess whether airway protection and blood product transfusion are required under a restrictive criterion, and start the administration of vasoactive agents and antibiotic prophylaxis as soon as possible
In complete agreement: 69%; In partial agreement: 31%
Quality of evidence: C; Strength of the recommendation: 1
AVB is a medical emergency with a high risk of death, whose prognosis is related to the stage of liver cirrhosis determined through the Child-Turcotte-Pugh (CTP) score, the Model for End-Stage Liver Disease (MELD) score, and the HVPG. A HVPG > 20 mmHg and a CTP class C score > 10 points is associated with failed treatment and risk of bleeding recurrence. Regarding in-hospital death, a MELD score > 19 predicts a 20% mortality rate and a score < 11 predicts a mortality rate below 5%.37,38
The initial management of the patient with liver cirrhosis and variceal bleeding should be centered on hemodynamic stabilization to maintain tissue perfusion, achieve optimum hemoglobin levels, and prevent infections and HE. Vasoactive drug administration should be started as soon as variceal gastrointestinal bleeding is suspected and combined with endoscopic examination within the first 12 h of hospital admission.39
Working group 2. Fluid resuscitation and initial pharmacologic therapyStatement 3: Intravascular volume replacement, with a restrictive resuscitation strategy with balanced crystalloid solutions is recommended, given that it has been associated with a decrease in adverse events and death
In complete agreement: 94%; In partial agreement: 6%
Quality of evidence: B; Strength of the recommendation: 1
In the first contact with the patient, venous accesses of adequate caliber and functionality should be obtained, to correct the hypovolemia. Intravascular volume replacement should be carried out early and at an adequate infusion velocity. The volume replacement considered optimum is a subject of debate, given that different factors must be taken into account that can modify the consequences of the intervention. The goal is sufficient fluid resuscitation. The principle of not doing harm through resuscitation is based on tolerance to fluids. Over-replacement can cause a decline in respiratory function, exacerbate bleeding, and increase portal pressure, oxygen deprivation, coagulation disorders, and hypothermia. This is thought to be due to modifications in the natural compensation mechanisms, causing vasodilation, coagulation factor dilution, increased blood vessel pulse pressure, and impaired clot formation, leading to a state of hemodilution and coagulopathy. Therefore, restrictive volume replacement offers greater benefits. Traditional parameters that have been utilized to measure resuscitation success are urinary volume, systolic arterial pressure, and central venous pressure. Point-of-care ultrasound (POCUS) may be used as a dynamic and useful instrument, as long as it does not involve a significant delay in emergency management and can be efficiently applied at various resuscitation times. Restrictive strategy signifies the search for permissive hypotension values (systolic arterial pressure of 80-90 mmHg or mean arterial pressure of 50-60 mmHg) and urinary volume > 40 ml/h, in addition to central venous pressure between 5-12 mmHg.40–42
The choice of the type of intravenous solution is based on its composition, compared with that of plasma. Advantages that balanced crystalloid solutions (e.g., lactated Ringer’s solution, Hartmann’s solution, Plasma-Lyte A®) have over 0.9% sodium chloride saline solution are a lower frequency of AKI, less need for renal replacement therapy, fewer cases of hyperchloremic metabolic acidosis, and a lower mortality rate.43 Colloids have shown higher rates of adverse events, such as AKI, and the need for renal replacement therapy and blood product transfusions, but with no impact on the mortality rate. Their use in AVB should be limited to patients that present with AKI or hepatorenal syndrome, who require the specific management of those complications.44
Statement 4: Volume replacement with intravenous solutions is an initial and temporary measure that can be complemented with blood product administration, if necessary
In complete agreement: 82%; In partial agreement: 6%; In partial disagreement: 6%; In complete disagreement: 6%
Quality of evidence: C; Strength of the recommendation: 2
The goals of initial management in the context of acute gastrointestinal bleeding are to correct the hypovolemia, control bleeding, and prevent bleeding recurrence, as well as associated complications and death.45 This is achieved through preserving tissue perfusion, signifying that every treatment stage is of vital importance as part of overall management. First contact with the patient occurs at different times from the start of bleeding, and so consequences are identified at different stages. Part of the complexity of volume resuscitation is precisely due to the fact that it is an immediate and emergency measure upon first contact. The strategy should be accompanied by the other measures aimed at achieving the abovementioned goals. The best manner to prevent adverse events from intravenous solution administration is by containing the bleeding and restoring the volume with blood products that can carry out the functions of hemostasis or tissue perfusion. Once blood products are available, resuscitation should include them, according to individual requirements and biochemical and hemodynamic goals. Importantly, transfusions should not be based solely on hemoglobin levels, but rather on the behavior of volume and bleeding, especially in patients with hemodynamic instability or inadequate oxygen transport.11
Once packed red blood cell transfusion has been decided upon, it should be carried when the restrictive transfusion threshold of a hemoglobin level < 7 g/dl is reached. The only general context in which the threshold for hemoglobin should be higher is in patients with acute coronary syndrome (<8 g/dl), underlying cardiovascular disease, or instability that justifies higher objectives. The maintenance goal for hemoglobin levels is 7-8 g/dl. In the context of upper gastrointestinal bleeding, compared with the strategy of liberal transfusion, restrictive transfusion is associated with better survival rates and fewer adverse events. In patients with Child-Pugh A and B cirrhosis, the benefit regarding mortality increases significantly, but not in patients with Child-Pugh C cirrhosis. The study demonstrating those findings also showed that the number of transfused units was lower in the group with the restrictive strategy.11,21,46,47
Statement 6: The administration of platelets should be decided upon according to individual cases because platelet count has not been associated with the risk of variceal bleeding or failure in controlling the bleeding or bleeding recurrence
In complete agreement: 81%; In partial agreement: 19%
Quality of evidence: C; Strength of the recommendation: 1
Systematic transfusion of platelets is not indicated but some authors suggest its use when levels fall below 50,000/mL. The intention of that strategy is directed at achieving hemostasis through coagulopathy correction. However, coagulopathy is not effectively corrected through that measure, added to the fact that it can favor volume overload and an increase in portal pressure.48 There is not enough evidence for recommending platelet transfusion in the context of AVB, nor has the intervention shown benefits. A platelet level ≥ 50,000/mL is considered sufficient for maintaining thrombin production. In addition, patients with cirrhosis have elevated levels of von Willebrand factor, increasing the adhesive capacity of platelets, and consequently, their greater effectiveness.49
Statement 7: The administration of fresh frozen plasma (FFP) or cryoprecipitates should be decided upon on an individual basis because fibrinogen levels or the INR have not been associated with the risk of variceal bleeding, failed bleeding control, or bleeding recurrence
In complete agreement: 75%; In partial agreement: 25%
Quality of evidence: C; Strength of the recommendation: 1
The INR has not been shown to be a marker of hemostasis status in the context of AVB. Despite being a marker of organic functioning in cirrhosis, its correction has no impact on coagulation status. Therefore, systematic FFP transfusion is not indicated in the presence of that biochemical alteration. Its use can favor volume overload and increase portal pressure, without correcting the coagulopathy.11,48
Thrombin generation or the endogenous thrombin potential (ETP) is considered a more reliable test than the INR, prothrombin time (PT), or activated partial thromboplastin time (aPTT) for reflecting coagulation balance. The administration of FFP in patients with liver cirrhosis and coagulopathy, defined as a PT/INR ratio ≥ 1.5, improved the INR, PT, and aPTT parameters 33.7%, 23.5%, and 16.6%, respectively (p < 0.0001). However, despite that improvement in the conventional coagulation parameters, FFP administration only improved ETP levels in 5.7% of patients, and more than 90% of them had normal-range baseline thrombin, evaluated in the thrombomodulin test (ETP-TM).50
Similarly, fibrinogen levels have not been shown to be correlated with failed bleeding control or recurrent bleeding.11,48 Despite the fact that low levels of fibrinogen (100 mg/dl) can reflect disease severity, its correction with cryoprecipitates does not affect survival or complications related to bleeding. However, fibrinogen replacement should be considered in patients with refractory AVB, if values are below 100 mg/dl.51
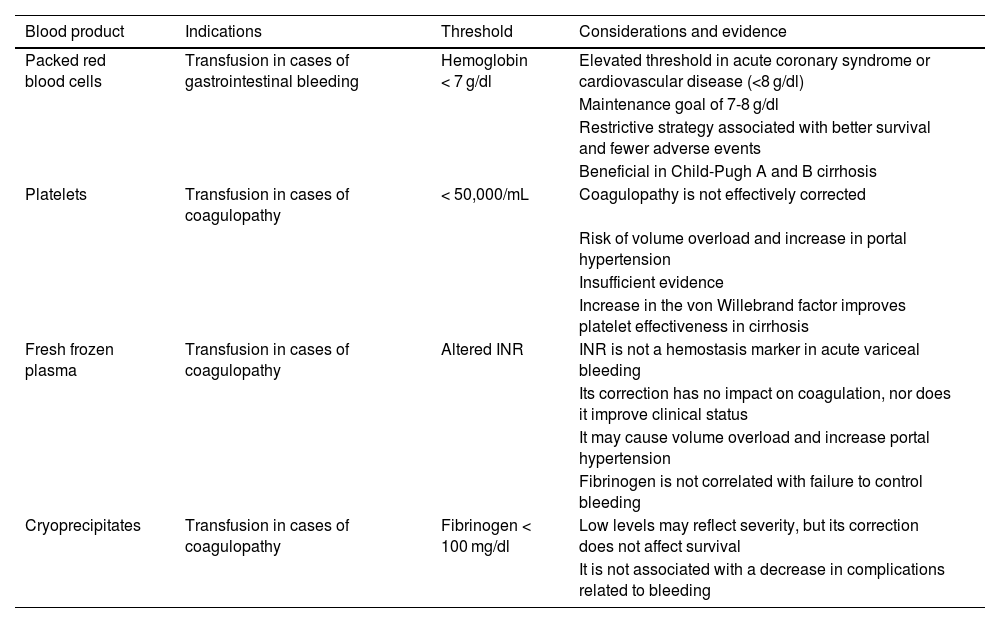
The use of FFP as a volume expanding agent has not been shown to be superior to other expanders (crystalloids and colloids).44 In the context of AVB, their administration has also been associated with increased mortality at 42 days (OR 9.41, 95% CI 3.71-23.90), failed bleeding control within the first 5 days (OR 3.87, 95% CI 1.28-11.70), and hospital stay above 7 days (OR 1.88, 95% CI 1.03-3.42).52Table 3 summarizes the indication for blood products in variceal upper gastrointestinal bleeding.
Statement 8: At this stage, resuscitation should be centered on goals at different levels:
- a)
Clinical and hemodynamic goals: correcting the hypovolemia, stopping the bleeding as soon as possible, and maintaining systolic arterial pressure > 90 mmHg and heart rate < 100 bpm is recommended
- b)
Laboratory goals: maintaining hemoglobin at 7-8 g/dl and lactate at normal values is recommended
In complete agreement: 82%; In partial agreement: 12%; In partial disagreement: 6%
Quality of evidence: C; Strength of the recommendation: 1
Blood products used in variceal upper gastrointestinal bleeding.
| Blood product | Indications | Threshold | Considerations and evidence |
|---|---|---|---|
| Packed red blood cells | Transfusion in cases of gastrointestinal bleeding | Hemoglobin < 7 g/dl | Elevated threshold in acute coronary syndrome or cardiovascular disease (<8 g/dl) |
| Maintenance goal of 7-8 g/dl | |||
| Restrictive strategy associated with better survival and fewer adverse events | |||
| Beneficial in Child-Pugh A and B cirrhosis | |||
| Platelets | Transfusion in cases of coagulopathy | < 50,000/mL | Coagulopathy is not effectively corrected |
| Risk of volume overload and increase in portal hypertension | |||
| Insufficient evidence | |||
| Increase in the von Willebrand factor improves platelet effectiveness in cirrhosis | |||
| Fresh frozen plasma | Transfusion in cases of coagulopathy | Altered INR | INR is not a hemostasis marker in acute variceal bleeding |
| Its correction has no impact on coagulation, nor does it improve clinical status | |||
| It may cause volume overload and increase portal hypertension | |||
| Fibrinogen is not correlated with failure to control bleeding | |||
| Cryoprecipitates | Transfusion in cases of coagulopathy | Fibrinogen < 100 mg/dl | Low levels may reflect severity, but its correction does not affect survival |
| It is not associated with a decrease in complications related to bleeding |
INR: international normalized ratio.
Clinical, hemodynamic, and laboratory parameters should be constantly evaluated and therapeutic strategies adapted to symptom progression. Fluid resuscitation, transfusion support, and vasoactive drug administration are initial measures, in addition to early endoscopy (within 12 to 24 h), for performing opportune endoscopic therapy and stopping the bleeding.
Hemodynamic parameters can be a marker of treatment response, although beta-blocker use should be considered because they can modify arterial pressure and heart rate. One of the goals is to maintain systolic arterial pressure > 90 mmHg and heart rate < 100 beats per minute, together with laboratory parameters, such as hemoglobin level.48 Increased lactate levels have been associated with unfavorable outcomes, such as longer hospital stay, admission into the intensive care unit, and death. In AVB, specifically, higher levels of lactate are associated with lower survival rates, a higher rate of admission into the intensive care unit, risk of bleeding recurrence, and need for transfusion. Its measurement is accessible and may be identified as a dynamic parameter in decision-making, by being a surrogate marker for tissue hypoxia.53
Statement 9: The administration of vasoactive drugs (terlipressin or octreotide) improves the parameters related to bleeding control and recurrence, required transfusions, and death
In complete agreement: 88%; In partial agreement: 6%; In partial disagreement: 6%
Quality of evidence: A; Strength of the recommendation: 1
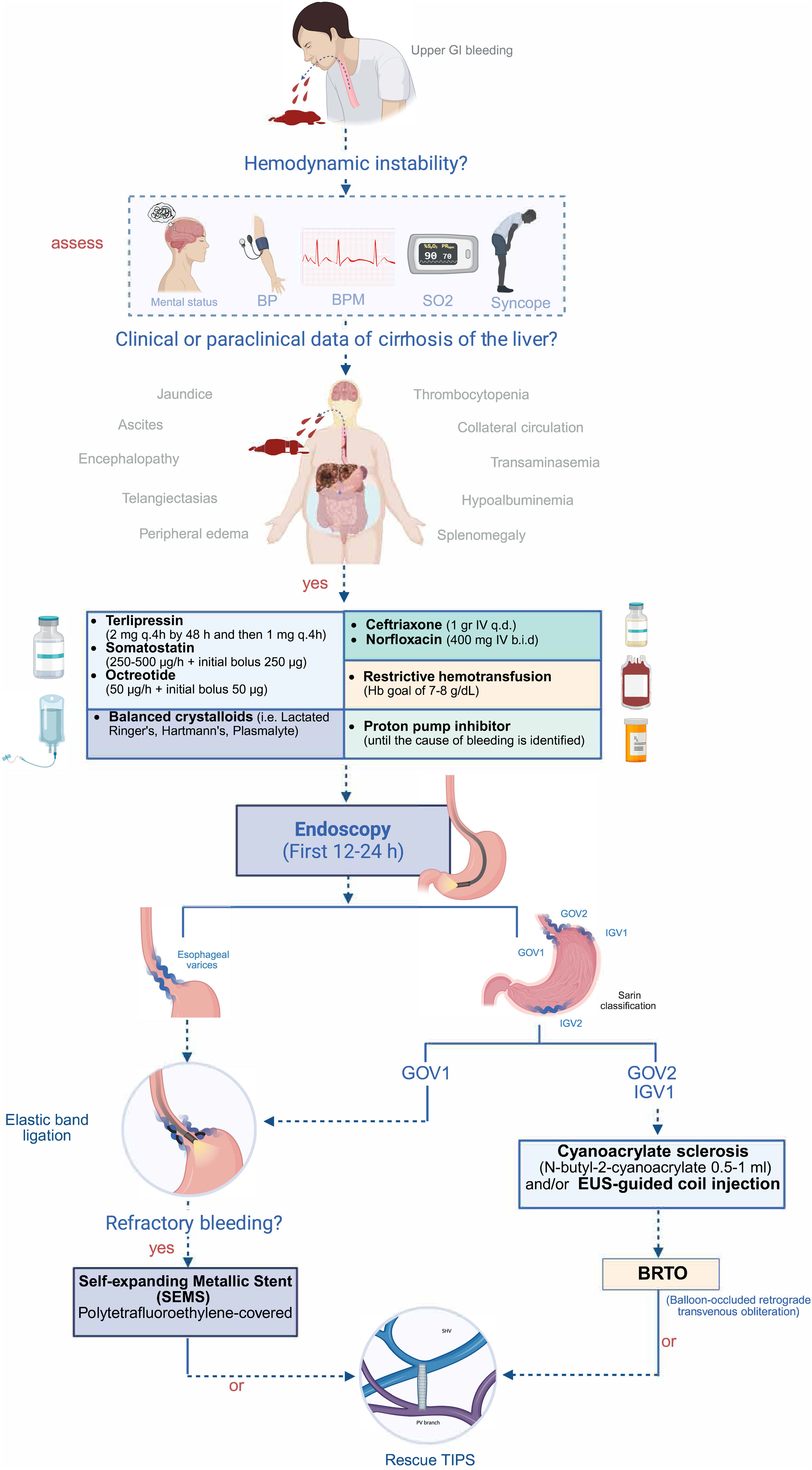
In the bleeding containment goal, the concomitant administration of vasoactive drugs with the rest of management is a crucial piece in the treatment of AVB. There must be a sufficient level of suspicion of a variceal origin for utilizing vasoactive agents, and they may be given when there is a confirmed or referred history of cirrhosis with PHT or clinical symptoms characteristic of liver disease. Once a variceal etiology is suspected, opportune treatment should be implemented, to efficaciously control portal pressure and variceal pressure, achieving hemostasis and reducing the mortality risk, along with performing therapeutic endoscopy.54 The bleeding recurrence rate decreases through the administration of those drugs.55 Treatment duration should be 2-5 days and combined with mechanical hemostasis strategies, such as elastic band ligation or sclerosing agent injection. Once therapeutic endoscopy is performed, extending pharmacologic therapy up to 5 days is debatable and has shown no superiority over shorter cycles, especially in patients with optimum liver function and no risk factors for recurrence, in whom the use of short cycles may be considered.9,54Fig. 1 describes the most frequently used vasoactive drug doses.
Bleeding control, bleeding recurrence, and mortality rates were similar, in the comparison of octreotide with terlipressin. However, related adverse events were more frequent with terlipressin (chest pain, abdominal pain, diarrhea, hyponatremia).56
Drugs that modify the natural course of upper gastrointestinal bleeding should be included in the initial management of the disease. Proton pump inhibitor use is indicated in any patient with upper gastrointestinal bleeding, regardless of prior documentation of liver disease with PHT, because up to 30% of gastrointestinal bleeds in that setting are due to nonvariceal causes. Therefore, initial drug management should include proton pump inhibitors. Once the origin of the gastrointestinal bleeding is determined to be variceal, proton pump inhibitors should be suspended, given that they provide no additional benefits and are associated with adverse events in patients with cirrhosis.57
Despite the advances made in treatment strategies, such as adequate fluid resuscitation, vasoactive drug use, and endoscopic therapy, the mortality rate at 6 weeks after an episode of AVB is 15-25%, due to different factors that are directly or indirectly related to the bleeding.17 Bleeding recurrence, complications associated with the bleeding, and hepatic and extrahepatic organ injury should be prevented at the same time as hypovolemia correction and bleeding control. In this management phase, fatal outcome predictive factors include bleeding severity, active or recurrent bleeding despite treatment, liver disease severity, kidney injury, cardiorespiratory deterioration, and the presence of infection.45 Prophylaxis with antibiotics, implemented as soon as possible upon suspecting a variceal origin, has been shown to reduce bacterial infections, bleeding recurrence, and the mortality rate,48,58–60 especially in patients with liver cirrhosis in Child-Pugh C.46,61 The antibiotic of choice is ceftriaxone (1 g every 24 h). Treatment duration should be short and not go beyond 7 days. Once the bleeding is resolved and the vasopressor has been withdrawn, suspending the antibiotic should be considered. Its use should not be continued after the patient is released.46 In a recent retrospective cohort study, there was no significant difference regarding mortality, rebleeding, or bacterial peritonitis. Thus, a more individualized approach, taking into account liver function, the endoscopic treatment employed, and local bacterial resistance, has been suggested to be more adequate.62 Regimens based on norfloxacin, according to availability, have shown similar results to those of ceftriaxone.
Coagulation disorders in patients with cirrhosis are complex and multifactorial (infection, endogenous heparin-like substances, kidney injury, endothelial dysfunction, and inadequate liposoluble vitamin absorption). Vitamin K is necessary for producing coagulation factor activation, making it a potential agent in the correction of coagulation disorders. Despite the apparent syllogism, patients with cirrhosis keep a peculiar balance between an altered coagulation status and a prothrombotic one. In the context of variceal upper gastrointestinal bleeding, vitamin K administration has shown no benefits in the treatment goals for bleeding, such as control, recurrence prevention, death, or required transfusion. Instead, the LIVER-K study showed that patients receiving vitamin K had a higher rate of required transfusions, a higher rate of poor prognosis from bleeding in endoscopy requiring endoscopic therapy, a higher rate of admission into the intensive care unit, longer hospital stay, and bleeding recurrence. Causality was not established due to the retrospective design of the study. Patients that receive vitamin K are thought to have more severe disease, which explains the outcomes observed. However, it did confirm that vitamin K does not modify treatment response in any way, and opened the possibility that it can worsen it.49
Statement 13: The administration of drugs, such as coagulation factors, etamsylate, or tranexamic acid, does not improve the parameters related to mortality, bleeding control or recurrence, or required transfusions
In complete agreement: 94%; In partial disagreement: 6%
Quality of evidence: B; Strength of the recommendation: 1
The HALT-IT study included a good number of patients with liver disease (> 40% per group) and compared the administration of tranexamic acid with placebo in upper and lower gastrointestinal bleeding. That drug was found to not modify the course of the disease or the mortality rate, but it increased the risk of venous thromboembolism.63
A recent single center randomized clinical trial (RCT) demonstrated that tranexamic acid administration reduced the failure to control bleeding by day 5, compared with placebo (6.3 vs 13.%, p = 0.006), and the failure to prevent rebleeding after day 5, up to 6 weeks, by preventing bleeding at the ligation site (12 vs 21.3%, p = 0.002). However, there was no significant difference in required blood product transfusions and no impact on mortality in 5 days or 6 weeks, in patients with DC (Child-Pugh B and C) and gastrointestinal bleeding.64
There is heterogeneous evidence on the use of activated recombinant factor VII. The benefits on modifying the course of the disease are marginal and due to its association with thrombotic events and high cost, its use is not justified in AVB.65 There is no evidence on the use of etamsylate in the management of gastrointestinal bleeding, and so in general, its use is not recommended.
Working group 3. The role of endoscopyClassification of esophageal varicesIn 1964, Brick and Palmer carried out the first attempt to classify esophageal varices through direct visualization, utilizing rigid esophagoscopy, and categorizing them as mild (<3 mm), moderate (3-6 mm), and severe (>6 mm).66 Several efforts to improve the classification of esophageal varices followed that initial attempt. In 1980, the Japanese Research Society for Portal Hypertension established general rules, unifying the descriptions of endoscopic findings and providing a more detailed macroscopic evaluation.67 Finally, in 1990, a simple classification system was maintained for diagnosing esophageal varices, dividing them into small (<5 mm) and large (> 5 mm), having determined as relevant the presence or absence of red spots.68 Semiquantitative measurement based only on appearance (esophageal varices flattened upon insufflation/confluents) was favored over a quantitative classification (millimetric through biopsy forceps),69 enabling a better estimation of their prevalence, better agreement, and a more adequate evaluation of the treatment to follow.
Child-Pugh C patients that present with large varices and red spots have been shown to have a greater risk of bleeding within the year following endoscopy. The size of esophageal varices determines the risk of bleeding, which is approximately 2% in patients without varices and increases to 15% in those with medium to large varices.70,71 Thus, the probability of bleeding can be determined through the risk indicators: size of varices (small or large), presence of red spots, and severity of cirrhosis (Child A compared with B-C).71
Classification of gastric varicesGastric varices are a complex collection of vascular shunts between the portosplenic venous system and the systemic veins of the abdomen and thorax.21 They are divided according to the Sarin classification, given that it is simple and correlated with the type of portosystemic collateralization developed by the patient. This classification divides gastric varices into 4 categories, depending on their location in the gastric cavity:72,73
- -
Type 1 gastroesophageal varices (GOV-1): extension of esophageal varices across the cardia toward the lesser curvature of the stomach
- -
Type 2 gastroesophageal varices (GOV-2): extension of esophageal varices beyond the cardia toward the greater curvature of the stomach and into the gastric fundus
- -
Type 1 isolated gastric varices (IGV-1): fundic varices that do not extend into the esophagus
- -
Type 2 isolated gastric varices (IGV-2): varices located in any part of the stomach, with the exception of the cardia and gastric fundus
GOV-1 are the most frequent type (approximately 75% of cases) but cardio-fundal varices (IGV-1 and GOV-2) have a higher incidence of bleeding, even with lower portal pressures, compared with esophageal varices or GOV-1, because of the presence of spontaneous portosystemic shunts (gastrorenal or splenorenal) and the consequent left circulation.73,74
Timing of endoscopy in acute variceal bleedingStatement 16: Esophagogastroduodenoscopy is ideally performed within the first 12 h of the patient’s clinical presentation, once adequate hemodynamic stabilization has been achieved
In complete agreement: 82%; In partial agreement: 12%; In partial disagreement: 6%
Quality of evidence: B; Strength of the recommendation: 1
Once the clinical, hemodynamic, and laboratory goals have been reached, diagnostic endoscopy should be performed. The timing of its performance is still controversial, given that the majority of studies evaluating it are observational and differ regarding the definitions of “early” or “late” endoscopy. A recent systematic review and meta-analysis reported a significant reduction in the mortality rate in patients that underwent endoscopy within the first 12 h after their clinical presentation (OR 0.56, 95% CI 0.33-0.95; p = 0.03).75 In contrast, another systematic review and meta-analysis reported a comparable general mortality rate (OR 0.72, 95% CI 0.36-1.46; p = 0.36) and a similar bleeding recurrence rate (OR 1.21, 95% CI 0.76-1.93; p = 0.41)76 in patients that underwent urgent and nonurgent procedures. Likewise, a cohort study that included 3,319 patients found no significant differences in outcome between patients that underwent endoscopy in the first 6-12 h and those that underwent the procedure within the first 24 h.77 The most recent international guidelines recommend the performance of endoscopy within the first 12 h,11,71,78,79 but under certain circumstances, its performance may be considered prudent within the first 24 h, stressing the need to achieve adequate hemodynamic stability in the patient before carrying out the endoscopic evaluation.
Treatment of acute bleeding due to esophageal varicesThe endoscopic diagnosis of AVB is made upon viewing a varix with active blood or signs of recent bleeding (white nipple sign or fibrin plug). The diagnosis can also be inferred if blood is detected in the stomach, with no other identifiable cause.78 Once the origin of the bleeding has been determined as variceal, endoscopic therapy must be performed according to the type of varices. In AVB due to esophageal varices, endoscopic band ligation (EBL) is the therapy of choice, followed by endoscopic sclerotherapy.11,71,78,80
EBL has 95.7% efficacy in the initial control of bleeding and a failure rate at 5 days of 7.1%.81 It is superior to sclerotherapy regarding the eradication of varices (RR = 1.06, 95% CI 1.01-1.12) and is associated with a lower risk for recurrent bleeding (RR 0.68, 95% CI 0.57-0.81) and complications (RR = 0.28, 95% CI 0.13-0.58), although with no significant differences in mortality (RR = 0.95, 95% CI 0.77-1.17).82 In contrast to this last result, a systematic review and meta-analysis more recently showed that EBL was associated with a lower mortality rate (9 RCTs, RR 0.72, 95% CI 0.54-0.97), compared with sclerotherapy.83
EBL has also been described to require fewer sessions for variceal obliteration (2.2 sessions less, 95% CI 0.9-3.5).78 EBL is a safe therapy, with only 2.6% of patients presenting with bleeding related to the procedure, even when the platelet count is below 50 × 109/l or when the INR is elevated (≥1.5).84
During the procedure, the first elastic band should be placed at the bleeding site, if located, and the remaining bands are placed working upwards in a helical fashion, with a maximum of 5 to 10 bands.78 Band placement after the collapse of varices is associated with an increased risk for recurrent bleeding, longer procedures, and a higher number of poorly placed bands.85,86 Factors associated with failed endoscopic treatment are age ≥ 60 years, MELD score ≥ 15 points, Child-Pugh C, presence of hepatocellular carcinoma, and systolic arterial pressure below 90 mmHg.87
Treatment of bleeding due to gastric varicesStatement 18: Endoscopic hemostasis, utilizing tissue adhesive (cyanoacrylate) obliteration of the gastric variceal types IGV-1 and IGV-2, as well as type GOV-2, is recommended. In bleeding due to GOV-1, ligation with elastic bands is recommended
In complete agreement: 94%; In partial agreement 6%
Quality of evidence: A; Strength of the recommendation: 1
EBL is the treatment of choice in AVB due to gastroesophageal varices in the lesser curvature of the stomach (GOV-1) because their vascular supply is similar to that of esophageal varices. In contrast, in AVB due to cardio-fundal varices (GOV-2 and IGV-1) that have a different vascular supply and are usually one diameter larger, obliteration with a tissue adhesive (N-butyl-2 cyanoacrylate) is indicated and can also be an option in AVB due to distal gastric varices (IGV-2). Splenic vein thrombosis is a common event in AVB caused by distal gastric varices, and so an imaging study should be carried out to classify the underlying vascular anatomy.21
The success of tissue-adhesive obliteration is influenced by the formulation of the cyanoacrylate utilized. The composition with 4 carbons (butyl) polymerizes rapidly, and with 8 carbons (octyl) polymerizes more slowly. N-butyl-2 cyanoacrylate can be applied in conjunction with lipiodol, a lipid agent, for the purpose of delaying polymerization, but the use of that agent is associated with the risk of embolization (0.7%) and so is not recommendable. Other associated complications may be: embolization of the tissue-adhesive thrombus, portal vein thrombosis, infection, injector trapped in the varix, or profuse bleeding at the time of glue extrusion.21,88
The hemostasis rate was superior with cyanoacrylate injection, compared with EBL (93.9 vs 79.5%) (p = 0.032; 95% CI 1.14-17.30), with an OR of 4.44 (95% CI 1.14-17.30).89 Similar results were shown in another meta-analysis, with a superior effective hemostasis rate (OR = 2.32, 95% CI 1.19-4.51) and a longer recurrent bleeding-free rate (HR = 0.37, 95% CI 0.43-1.02), with no need for a higher number of sessions for achieving obliteration.90 In addition, compared with other endoscopic techniques, cyanoacrylate injection was associated with a lower risk of death (RR 0.59, 95% CI 0.36-0.98) and recurrent bleeding (RR 0.49, 95% CI 0.35-0.68), with a similar adverse event rate.91
Once endoscopic therapy has been performed, control endoscopy should be carried out every 2-4 weeks until the gastric varices are completely obliterated.21
Statement 19: In the presence of active bleeding of gastric varices and no access to tissue adhesive, performing ligation with elastic bands is recommended, only when it is possible to completely suction the varix inside the ligating barrel cap and subsequently obliterate the varices, as soon as tissue adhesive is available
In complete agreement: 75%; In partial agreement: 25%
Quality of evidence: C; Strength of the recommendation: 2
In cases when cyanoacrylate injection therapy is not available, EBL is a temporary management option when facing this medical emergency.79,92 A clinical trial reported similar success rates, with respect to acute bleeding control, but bleeding recurrence was significantly higher in the EBL group (p = 0.044).93 In contrast, a meta-analysis that included 7 observational RCTs reported that cyanoacrylate injection was superior to EBL, regarding acute bleeding control (OR 2.32, IC 95% CI 1.19-4.51), and had a higher bleeding recurrence-free period (HR 0.37, 95% CI 0.24-0.56), reporting no significant differences in the risk of death or complications.90
Statement 20: Endoscopic hemostasis is recommended for gastric variceal obliteration, utilizing a tissue adhesive (cyanoacrylate), coils, or both methods, guiding the puncture through ultrasound endoscopy
In complete agreement: 75%; In partial agreement: 25%
Quality of evidence: C; Strength of the recommendation: 2
Ultrasound endoscopy (USE) enables the visualization of esophagogastric varices and other venous collaterals (paraesophageal, periesophageal, and perforating veins). The obliteration of gastric varices through USE has been shown to be efficacious, achieving high eradication rates and low recurrence and adverse event rates. It is currently the technique of choice in patients with AVB or with high-risk stigmata (diameter > 20 mm, cherry-red spots), using cyanoacrylate, coils, or their combination.94
If a coil is placed in a varix using USE and cyanoacrylate is later injected, it adheres to the fibers of the coil, preventing the cyanoacrylate from producing embolization.95
USE also enables the confirmation of adequate obliteration of the varices and feeding vessel and notably reduces the risk of an ectopic thrombus, increases the complete vascular embolization rate, reduces the need for repeat procedures, and lowers the bleeding recurrence rate.94,96
Kouanda et al. conducted a single-center observational study (n = 80) on patients with gastric varices and high-risk stigmata (mean size of 22.5 ± 4 mm and cherry-red spots), with no previous bleeding, who underwent USE with the combination of coil placement and cyanoacrylate injection. Technical success was achieved in 100% of the patients. Variceal obliteration was confirmed through USE in 96.7%, and 67.7% required only one session for achieving obliteration.97
Robles-Medranda et al. carried out a descriptive, prospective, observational study that evaluated the role of USE (coils + cyanoacrylate) in 30 patients with gastric varices. Technical success was achieved in 26 of the 30 patients, the mean number of coils used was 2 (1-3), and the mean volume of cyanoacrylate was 1.8 ml (1.2-2.4 ml).98
Current evidence shows that USE with the application of coils in combination with cyanoacrylate is a cost-effective tool in the treatment of AVB caused by gastric varices or with high-risk stigmata. It provides a high rate of technical and clinical success and adverse events are infrequent. It should be the initial therapeutic conduct at centers that have the resource.
Statement 21: Argon plasma coagulation is recommended as the first option of endoscopic hemostasis in patients with active bleeding due to portal hypertensive gastropathy. Hemostasis through cryotherapy with liquid nitrogen or hemostatic powders may be considered when argon plasma is not available or as rescue therapy
In complete agreement: 87.5%; In partial agreement: 12.5%
Quality of evidence: B; Strength of the recommendation: 1
Portal hypertensive gastropathy (PHG) refers to gastric mucosa with a mosaic or snakeskin pattern that is frequently seen in patients with cirrhosis and PHT. The presence of associated subepithelial bleeding differentiates severe hypertensive gastropathy from mild cases.71,99,100 PHG has been reported to have a prevalence of 49 to 80% of patients with cirrhosis, and their primary clinical manifestation is chronic anemia.101–103
In cases of bleeding due to PHG, argon plasma coagulation (APC) is the treatment of choice, with a success rate of up to 81% and a mean of 1-3 sessions.104 A prospective study showed that the successful hemostasis rate varied according to PHG distribution (OR 1.65, p = 0.004). A higher response rate was achieved in patients with exclusive involvement of the gastric body (68%), followed by 60% in patients with fundal involvement, and 40% in cases of pangastric involvement.105 Hemostatic powders may be useful when argon plasma is not available, or as a rescue measure.106,107 Another endoscopic therapy with reports of successful cases is cryotherapy with liquid nitrogen. However, its limited clinical evidence and scant availability (it is unavailable in Mexico) restrict its use.101,108
Gastric antral vascular ectasia (GAVE) is a vascular lesion characterized by the presence of red spots that can form into striations or bands, located proximal to the pylorus and converging there, with a “watermelon stomach” appearance.101 It is an important cause of the chronic anemia that presents in 3-26% of patients with cirrhosis, but can also manifest as acute bleeding.109,110
APC is the treatment of choice and has been shown to have a success rate of up to 86%, with a mean of 3.5 sessions, achieving fewer episodes of anemia and reducing the need for transfusions, without producing adverse events.111,112 An alternative to APC is EBL, which achieves a success rate of up to 90%, with a mean of 2.28 sessions.113 A recent meta-analysis compared EBL with thermal ablative therapies (APC and radiofrequency ablation), reporting better bleeding control (OR 4.48, 95% CI 1.36-14.77, p = 0.01) and a lower number of sessions (–1.44, 95% CI –2.54 to –0.34, p = 0.01) with EBL.114
Despite the good effectiveness of APC and EBL, approximately 15% of cases of GAVE are refractory to treatment. In such cases, radiofrequency ablation could be useful, with a success rate of 90%, a mean of 2 sessions, and mild adverse events, such as bleeding and superficial ulcers. However, it is an expensive tool with little accessibility.115
Other treatment options in patients with refractory GAVE are endoscopic mucosal resection and surgical antrectomy, which could play a role in patients with CC that are good candidates for surgical management.116–118
Working group 4. Strategies for managing failed bleeding controlStatement 23: In patients with persistent variceal bleeding within 5 days after initial hemostatic therapy, a second endoscopic treatment attempt or rescue/salvage TIPS placement may be carried out
In complete agreement: 94%; In partial agreement: 6%
Quality of evidence: A; Strength of the recommendation: 1
Refractory AVB is secondary to failed bleeding control or failed rebleeding prevention.119 Failure to control bleeding or persistent bleeding is defined as death or the need to modify treatment, when presenting with any of the following: 1) fresh hematemesis in ≤ 2 h after the start of treatment, the performance of endoscopic therapy, in patients with a nasogastric tube, or aspiration of >100 ml of fresh blood, 2) decrease in hemoglobin ≥ 3 g or of 9% in hematocrit within a 24 h period, or 3) the development of hypovolemic shock, considering the first 5 days as the acute bleeding episode. On the other hand, failure to prevent rebleeding (recurrent variceal bleeding) is defined as any clinically significant episode of rebleeding (secondary to PHT) after day 5. Clinically significant bleeding means the presence of recurrent melena or hematemesis resulting in hospital admission, blood transfusion, decrease of 3 g in hemoglobin, or death, in the following 6 weeks.120
Persistent bleeding occurs in approximately 12 to 23% of patients with AVB, resulting in an elevated mortality rate.121 Adequately defining when endoscopic treatment has failed is important for scaling a rescue/salvage therapy, which includes: balloon tamponade, the placement of self-expanding metal stents, and TIPS placement.122
TIPS placement is the therapy of choice in patients with failed medical and endoscopic treatment.11 It involves the creation of a radiologically-guided shunt, using a stent that directs portal flow directly to the hepatic vein through the liver, which decreases portal pressure.119 The placement of polytetrafluoroethylene (PTFE) stents is preferred because they have been shown to reduce the risk of HE and they maintain permeability, compared with uncovered stents.123 The rebleeding rate after rescue TIPS placement is approximately 15%,124 and the grade of ACLF is an important factor in rebleeding (OR 1.699, 95% CI 1.056-1.663, p = 0.040).125
Survival after rescue TIPS placement in low-risk patients (MELD score < 15 and lactate ≤ 2.5 mmol/l) is 86% at 6 weeks and 78% at one year.125 However, overall survival continues to be low, despite the implementation of EBL instead of sclerotherapy, and the use of covered stents. In a recent systematic review and meta-analysis, the short-term (4-6 weeks) and long-term (1 year) mortality rates were 33 and 46%, respectively.124 To achieve an improved prognosis, secondary prophylaxis, including preventive TIPS placement in high-risk patients, needs to be optimized.126
The stage of liver disease evaluated using the Child-Pugh classification predicts mortality at 6 weeks (OR 1.304, 95% CI 1.076-1.580, p = 0.007) and 12 months (OR 1.396, 95% CI 1.147-1.698, p = 0.001) in patients with refractory bleeding that underwent rescue TIPS placement.127 TIPS placement may be futile in patients with a Child-Pugh C score ≥ 14 points or a MELD score ≥ 30 points and lactate ≥ 12 mmol/l, unless there is the possibility of performing a liver transplant in the short term, with a mortality rate at 6 weeks above 90% in that group of patients.125,127
A second endoscopic therapy can be attempted if the patient is hemodynamically stable, the varices are susceptible to therapy, and there are no apparent local complications from previous endoscopic treatments. This is especially applicable if initial therapy was considered suboptimal (e.g., if it was performed under less-than-ideal circumstances or by an endoscopist without much experience). The decision to perform an additional endoscopic therapy should be individualized, given that there are no controlled studies that provide solid data on those modalities, in the context of persistent bleeding.119,122
Prior to TIPS placement, liver function should be taken into account and Child-Pugh C (< 14 points) and Child-Pugh B patients with active bleeding during the initial upper gastrointestinal endoscopy should be prioritized for the preventive implantation of a TIPS within 72 h after the bleeding event, because this approach significantly improves survival.128 In cases when, for logistical or other reasons, preventive TIPS placement is not feasible within that window of time, a recent meta-analysis suggests that the survival benefit is maintained up to 16 days in patients with Child-Pugh C and up to 6 days in patients with Child-Pugh B, who present with active bleeding.129 The little evidence there is suggests that the preventive TIPS strategy may be applied in patients with bleeding due to gastric varices.130
Statement 25: The placing of a self-expanding metallic stent (SEMS) in cases of persistent variceal bleeding is recommended and it should not be removed before rescue/salvage TIPS placement due to the high risk of recurrent bleeding
In complete agreement: 75%; In partial agreement 18%; In partial disagreement 7%
Quality of evidence: C; Strength of the recommendation: 2
Current clinical practice guidelines recommend the self-expanding metallic stent (SEMS), or if not available, a balloon tamponade, as bridging treatment until TIPS placement or definitive treatment is carried out. In the study by Pfisterer et al. that included 34 patients, 12 (35.3%) had failed pharmacologic and endoscopic treatment before SEMS implantation. The SEMS controlled the acute bleeding in 27 (79.4%) patients. Thirteen patients died with the SEMS in situ. After successful stent removal (20 patients), 7 (35%) patients had recurrence and 10 (29.4%) had no episode of bleeding in the following 6 weeks. Nearly half the patients died from bleeding-related complications and those authors emphasized the fact that none of them had early TIPS placement after SEMS.131
A meta-analysis that included a total of 155 patients showed that treatment success (defined as the absence of bleeding within 24 h after SEMS placement) was consistently elevated in all the studies analyzed. The pooled clinical success rate was 96% (95% CI 0.90-1.00), reported in the 12 studies included in the meta-analysis. However, 5 studies reported ineffective bleeding control, with failure to achieve hemostasis within the first 24 h, reporting the lowest clinical success rate of 78%.132 Another meta-analysis reported an estimated pooled rate for failed bleeding control of 0.18 (95% CI 0.11-0.29) and of rebleeding after stent removal of 0.16 (95% CI 0.04-0.48).133
The balloon tamponade with a Sengstaken-Blakemore (SB) tube, originally described in 1950 by Sengstaken and Blakemore,134 is a treatment option in bleeding due to subcardial gastric or esophageal varices (GOV-1, GOV-2). The device has two balloons (gastric and esophageal). The first is placed against the cardia and the second directly compresses the esophageal varices. The gastric balloon is inflated with 200 cc of air (4 syringes of 50 cc) and then softly pulled until positioned against the cardia. Afterwards, the esophageal balloon should be inflated with progressive quantities of air (customarily, 40 ml initially and then in increments of 10 ml each time), confirming the pressure after each quantity of air that is added, until reaching a pressure between 50 and 60 mmHg, with continuous monitoring of the pressure to prevent esophageal perforation. After esophageal balloon placement, the pressure must be verified every hour due to the risk of air leaks that can cause a loss of pressure. In such cases, small quantities of air must be added until the necessary pressure is recovered. The SB tube does not require traction and should be attached to facial devices. It is important to carry out a control X-ray to verify the correct location of the balloons. The efficacy of this type of device varies from 70 to 90% in primary hemostasis, although almost 50% of cases present with recurrence upon balloon deflation. Therefore, the use of this tool is recommended only as a transitory measure for fewer than 24 h, to then carry out a definitive treatment. The SB tube should be placed by experienced personnel to prevent complications, such as esophageal perforation or aspiration pneumonia.134,135
In 2018, Choi et al. reported 66 cases that underwent SB tube placement. The general initial hemostasis success rate was 75.8% and the independent factors associated with bleeding control were: a lack of mechanical ventilation support prior to tube placement (OR 8.5, p = 0.007) and a Child-Pugh score < 11 points (OR 8.5). The rebleeding rate was 22%, and there was esophageal perforation in 6.1%, with a 30-day mortality rate of 42.4%.136
Escorsell et al. conducted an RCT that compared the SB tube (n = 15) with the Danis SX-ELLA stent (n = 13), in patients with cirrhosis and massive or refractory variceal bleeding. The primary endpoint was treatment success, defined as survival at day 15 with bleeding control and no adverse events. There was a higher success rate in the esophageal stent group compared with the SB balloon tamponade (66 vs 2%, p = 0.025), as well as higher bleeding control (85 vs 47%, p = 0.037) and fewer adverse events (15 vs 47%, p = 0.077). There was no significant difference in survival at 6 weeks (54 vs 40%, p = 0.46), and aspiration pneumonia was the most frequent adverse event associated with the balloon tamponade.137
Self-expanding and covered metallic esophageal stents are more efficacious than balloon tamponade for the temporary control of massive or refractory variceal bleeding, with a lower adverse event rate. Both procedures are an option at hospital centers that do not have rapid access to TIPS.137 Even though esophageal stent placement is a safer and more effective option, balloon tamponade is a useful resource for recurrent bleeding control in places where there are no endoscopic methods or esophageal stents.
Working group 5. Secondary prophylaxis recommendationsCombination therapy as secondary prophylaxis after an AVB episode has been shown to be superior to monotherapy. In the study by Lo et al., recurrent bleeding due to esophageal varices occurred in 43% of patients in the nonselective beta-blocker (NSBB) group as monotherapy, compared with 26% in the NSBB plus EBL group (p = 0.07), with similar results in AVB due to gastroesophageal varices (p = 0.05), but with no impact on mortality.138
Those results were confirmed by a later meta-analysis of individual data, in which the combined therapy (NSBB + EBL) reduced the risk of rebleeding at all stages of liver disease (Child-Pugh A-C), compared with EBL as monotherapy. In addition, in patients in stages B and C, the combined therapy significantly reduced the risk of death (incidence rate: 0.46, 95% CI 0.25-0.85, p = 0.013).139
In contrast to EBL, treatment with a beta-blocker was reported to reduce portal pressure and prevent the development of complications other than rebleeding. Patients with a hemodynamic response (decrease in the HVPG ≥ 20% from the baseline HVPG or < 12 mmHg) and no ascites at the time of bleeding had a lower risk of developing ascites or HE (OR 0.35, 95% CI 0.22-0.56), there was a reduced risk for developing other complications, and patients with ascites had a significant reduction in the risk of refractory ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome (OR 0.27, 95% CI 0.16-0.43). Treatment with a beta-blocker also reduced the risk of death and the need for liver transplantation.140
Carvedilol is more potent in reducing the HVPG than traditional beta-blockers,141 achieving a hemodynamic response in nearly 75% of cases. In addition, it reaches its maximum effect on portal pressure at low doses (12.5 mg/day) that are better tolerated than the effective doses required with other NSBBs. Because of those advantages, it is the most widely used beta-blocker.142 The recommended initial dose is 3.125 mg twice a day, titrated up to 6.25 mg twice a day (12.5 mg/day), avoiding or discontinuing its use in patients with ascites that develop arterial hypotension and/or kidney dysfunction.143 Along with carvedilol’s greater effectiveness in preventing rebleeding, its administration has been shown to be superior to propranolol in reducing the risk of other decompensations, such as the development or worsening of ascites.141,144
Statement 27: The placement of a transjugular intrahepatic portosystemic shunt (TIPS) should be considered in patients with failed first-line secondary prophylaxis (nonselective beta-blocker plus endoscopic ligation)
In complete agreement: 81.3%; In partial agreement: 18.8%
Quality of evidence: A; Strength of the recommendation: 1
In a study with a mean 23-month follow-up period that included non-selected patients with cirrhosis who received successful endoscopic hemostasis for variceal bleeding, the covered TIPS was superior to the endoscopic ligation plus NSBB for reducing variceal bleeding recurrence (0 vs 29%, p = 0.001), although it did not improve survival. The TIPS was associated with higher rates of early HE (35 vs 14%, p = 0.035), but that difference decreased during the long-term follow-up (38 vs 23%, p = 0.121).145
The primary risk factors for developing HE include age > 65 years, Child-Pugh score > 12, previous HE, placement of a large-diameter stent (> 10 mm), and a low portosystemic pressure gradient (PPG < 5 mmHg). The risk of HE may surpass the potential benefit of the procedure in patients presenting with those risk factors.146
TIPS placement is indicated for the prevention of variceal bleeding recurrence and for treating refractory ascites. It also reduces the incidence of additional decompensation events, compared with standard treatment, and increases survival in highly selected patients.147
Statement 28: In patients with gastric varices and contraindications for TIPS placement, such as spontaneous episodes of hepatic encephalopathy, balloon-occluded retrograde transvenous obliteration (BRTO) may be considered a treatment option in selected patients
In complete agreement: 81.3%; In partial agreement: 18.8%
Quality of evidence: C; Strength of the recommendation: 2
The following contraindications are considered absolute for TIPS placement: West Haven (WH) grade III or IV HE, serum bilirubin > 5 mg/dl, septicemia, or uncontrolled bacterial infection, severe pulmonary hypertension (mean pulmonary arterial pressure [mPAP] > 45 mmHg), right-sided heart failure, or congestive heart failure with an ejection fraction < 40%, heart valve disease (especially aortic stricture), and obstructive cholestasis. The following are considered relative contraindications: WH grade I or II HE, serum bilirubin between 3-5 mg/dl, moderate pulmonary hypertension (mPAP 35-45 mmHg), coagulopathy, (INR > 5, platelets < 20,000/mL), and hepatocellular carcinoma.128
Balloon-occluded retrograde transvenous obliteration (BRTO) is a minimally invasive procedure based on the sclerosing of gastric varices. It has the advantage of being feasible and successful in patients with poor hepatic functional reserve or hemorrhagic diathesis. Splenorenal shunt occlusion could increase portal pressure and the risk of complications other than variceal bleeding, such as ascites. An important disadvantage is that BRTO is a relatively new treatment modality that has not yet been widely implemented and may not always be available.148
ConclusionsThis first Mexican Consensus on Acute Variceal Bleeding, developed using the RAND/UCLA process and supported by the Delphi methodology, has produced a set of practical recommendations, whose aim is to standardize the management of this emergency in patients with cirrhosis of the liver in Mexico. The present consensus provides information on the approach to and treatment of acute variceal bleeding and emphasizes the crucial role of an early and detailed evaluation of the patients, as well as the implementation of specific management strategies at each treatment stage, from initial resuscitation to endoscopic management and secondary prophylaxis (Fig. 1).
This consensus represents a significant advance in the standardization of variceal bleeding management in Mexico and lays the groundwork for future research directed at improving the clinical results and quality of life of patients with cirrhosis and complications of PHT.
Financial disclosureNo type of external funding was received. The resources for the development of this publication were entirely provided by the AMG.
Ethical considerations- -
Protection of persons and animals. No experiments were conducted on humans or animals in this research study.
- -
Data confidentiality. All protocols of our work center were followed, guarding the privacy and confidentiality of all study participants, preserving the anonymity of the data of all subjects evaluated.
- -
Right to privacy and informed consent. Given that the present manuscript includes no patients or subjects, informed consent was not required.
F. Higuera de la Tijera is a speaker for Grünenthal, IPSEN, Roche, Abbott, Ferrer, and Medix; J.A. Velarde Ruiz Velasco has been a speaker for Megalabs, Medix, Alfasigma, and IPSEN; E. Cerda Reyes is a speaker for CellPharma, IPSEN, Alfasigma, Malloly-Spindler, and Medix; A. Bautista Santos is a speaker for Roche; R. Moreno Alcántar is a speaker for IPSEN and Grünenthal; G. E. Castro Narro is a speaker for Medix and IPSEN; A. Noble Lugo is a speaker for Alfasigma, Grünenthal, Adium, Astra Zeneca, Ferrer, Chinoin, Liomont, and M8; O.E. Trujillo Benavides is a speaker for Medix, Carnot, Asofarma, Chinoin, and Grünenthal; Y. M. Velasco Santiago is a speaker for Mayoly-Spindler; N. J. Fernández-Pérez is a speaker for Grünenthal, Medix, and Megalabs; J. L. Pérez Hernández is a speaker for Grünenthal; J. Crespo is a speaker for Megalabs and Alfasigma; Edgar Santino García Jiménez is a speaker for Megalabs and Alfasigma; A. J. Montaño-Loza, J. M. Aldana Ledesma, A. D. Cano Contreras, L. F. De Giau Triulzi, J. Cerna Cardona, and D. K. Tapia declare they have no conflict of interest.