In capsule endoscopy (CE) performed on patients with confirmed Crohn’s disease, the video capsule is retained in up to 13% of cases,1 motivating the development of a patency capsule (PC) (Given Imaging, Yokneam, Israel). The PC is a biodegradable capsule, with a diameter similar to that of the PillCam SB3 video capsule, that begins to degrade 30h after its ingestion, enabling it to pass through a stricture that has caused its retention.2 Symptomatic PC retention is a rare complication, with few descriptions in the literature. It is characterized by transitory obstructive symptoms in the majority of cases.3 A multicenter study including 1615 patients that underwent a PC test reported symptomatic retention in 20 patients 1.2%), only one of which required surgery. The rest of the cases resolved spontaneously or after corticosteroid therapy.4 The case of a patient with intestinal obstruction secondary to PC ingestion is presented herein.
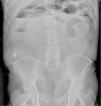
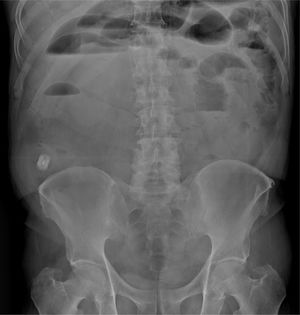
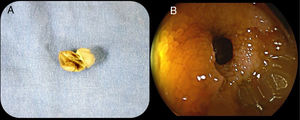
A 68-year-old man with a diagnosis of Crohn’s disease of 9-year progression had a history of 2 episodes of bowel obstruction secondary to the disease. He was currently asymptomatic and under treatment with mesalazine, azathioprine, and adalimumab. CE was ordered to evaluate treatment response. Before the CE, the patient underwent a PC test. Six hours after PC ingestion, he presented with colicky abdominal pain and bloating and was unable to pass gas. A plain abdominal x-ray revealed the presence of the PC, intestinal segment dilation, and air-fluid levels (Fig. 1). Twelve hours after symptom onset, the patient had 6 liquid stools, with an important reduction of pain and bloating. Fifty hours after ingestion, the patient expelled a deformed PC. A stricture of the terminal ileum situated 50cm from the ileocecal valve was identified through retrograde enteroscopy (Fig. 2).
Symptomatic PC retention is a rare complication, but thanks to its process of degradation, it is usually transitory. The PC test should be carried out on all patients suspected of small bowel stricture to prevent the video capsule from being retained during CE or causing obstruction that could require emergency surgery.
Ethical considerationsFor the publication of the present article, the lead author requested and obtained the patient’s informed consent. The work followed the current regulations in bioethical research and was authorized by the institutional ethics committee. The authors declare that this article contains no personal information that could identify the patient.
Financial disclosureNo financial support was received in relation to the present article.
Conflict of interestGerardo Blanco-Velasco is a speaker for Medtronic. The rest of the authors declare they have no conflict of interest.
Please cite this article as: Blanco-Velasco G, Ramos-García J, Zamarripa-Mottú R, Solórzano-Pineda OM, Hernández-Mondragón OV. Retención sintomática de la cápsula Patency en un paciente con enfermedad de Crohn confirmada. Revista de Gastroenterología de México. 2020;85:370–372.