Acute pancreatitis (AP) and recurrent acute pancreatitis (RAP) are conditions, whose incidence is apparently on the rise. Despite the ever-increasing evidence regarding the management of AP in children and adults, therapeutic actions that could potentially affect having a poor prognosis in those patients, especially in the pediatric population, continue to be carried out. Therefore, the Asociación Mexicana de Gastroenterología convened a group of 24 expert pediatric gastroenterologists from different institutions and areas of Mexico, as well as 2 pediatric nutritionists and 2 specialists in pediatric surgery, to discuss different aspects of the epidemiology, diagnosis, and treatment of AP and RAP in the pediatric population. The aim of this document is to present the consensus results. Different AP topics were addressed by 6 working groups, each of which reviewed the information and formulated statements considered pertinent for each module, on themes involving recommendations and points of debate, concerning diagnostic or therapeutic approaches. All the statements were presented and discussed. They were then evaluated through a Delphi process, with electronic and anonymous voting, to determine the level of agreement on the statements. A total of 29 statements were formulated, all of which reached above 75% agreement in the first round of voting.
La pancreatitis aguda (PA) y la pancreatitis aguda recurrente (PAR) son enfermedades cuya incidencia aparentemente va en incremento. A pesar de la creciente evidencia sobre el manejo de la PA en adultos y niños, aún se siguen teniendo conductas que potencialmente podrían impactar en un pronóstico no tan adecuado de estos pacientes, sobre todo en población pediátrica. Es por esto que la Asociación Mexicana de Gastroenterología convocó a un grupo de 24 gastroenterólogos pediatras expertos, de diferentes instituciones y de diferentes zonas geográficas de México, 2 nutriólogas pediatras, así como 2 especialistas en cirugía pediátrica para discutir sobre diferentes aspectos de la epidemiología, diagnóstico y tratamiento de la PA y PAR en población pediátrica. El objetivo de este documento es presentar los resultados obtenidos de este consenso. Se organizaron 6 mesas de trabajo con diferentes tópicos sobre la PA. Cada mesa de trabajo revisó la información y generó los enunciados/declaraciones que consideraron pertinentes para cada módulo, en tópicos que representaran recomendaciones o puntos de debate sobre cuestiones de abordaje diagnóstico o terapéutico. Se presentaron y discutieron todas las declaraciones. Posteriormente, se sometieron a evaluación mediante un proceso Delphi, de voto anónimo, vía electrónica, para conocer el nivel de acuerdo en los enunciados/declaraciones. Se elaboraron un total de 29 enunciados/declaraciones. Todas las declaraciones tuvieron un porcentaje de consenso mayor del 75% en la primera ronda de votación.
Acute pancreatitis (AP) is defined by the presence of at least 2 of the following criteria: abdominal pain consistent with AP, serum amylase and/or lipase levels ≥ 3-times higher than the upper limit of normal, and imaging findings of AP. Recurrent acute pancreatitis (RAP) is defined as the presentation of 2 episodes of AP, with an interval between them of at least 4 weeks of clinical symptom resolution and pancreatic enzyme normalization.1 AP is classified as: mild, when resolved within one week, with no local or systemic involvement; moderate, when associated with organ dysfunction not longer than 48 h; and severe, when there is multiple organ failure for more than 48 h.2
Despite increasing evidence regarding AP management in children and adults, therapeutic actions are still being carried out that can potentially affect having a poor prognosis in those patients, especially in the pediatric population. Therefore, the Asociación Mexicana de Gastroenterología (AMG) convened a group of experts to discuss the different aspects of the epidemiology, diagnosis, and treatment of AP and RAP in the pediatric population. The aim of this document is to present the consensus results.
Material and methodsTwenty-four pediatric gastroenterologists belonging to the AMG, from different institutions and regions of Mexico, were initially convened, along with 2 pediatric nutritionists and 2 specialists in pediatric surgery. Six working groups were organized to address different topics on AP. Each working group was made up of 3 to 6 experts, one of whom was the coordinator, in each group. An initial information search was carried out on the following databases: CENTRAL (the Cochrane Central Register of Controlled Trials), MEDLINE (PubMed), and EMBASE (Ovid), encompassing the time frame of January 1, 1990 to June 30, 2022. The bibliographic search criteria were: “pancreatitis”, “children pancreatitis”, “acute pancreatitis, “recurrent pancreatitis”, “recurrent acute pancreatitis”, “severe acute pancreatitis”, “acute pancreatitis guidelines”, “acute pancreatitis children”, “management acute pancreatitis children” and their Spanish equivalents. All publications in English and Spanish were identified (original articles, consensuses, guidelines, systematic reviews, and meta-analyses), as well as publications the coordinators and members of the consensus groups considered relevant, and they were shared with all the participants. The first meeting was held virtually to explain the dynamics of the endeavor. Each working group reviewed the information and formulated the statements considered pertinent for each module, on topics that involved recommendations or points of debate related to diagnostic or therapeutic approaches. Each of the coordinators sent the statements, with a brief justification of each of them, to the general coordinators of the consensus (RVF and YRS), who then put them together and sent them to all the participants to read. All the statements were presented and discussed at a second virtual meeting. The statements were evaluated through a Delphi process, with electronic anonymous voting, to determine the level of agreement on the statements; each statement was evaluated on a 3-point Likert scale: a) in agreement, b) in disagreement, and c) in abstention. After a first round of voting, the statements that achieved consensus (over 75% agreement) were accepted. The statements that did not achieve consensus (under 75% agreement) were re-evaluated, and either eliminated or reformulated by the corresponding working group, after which they were submitted to a second presentation round, and underwent a second anonymous voting round, applying the same criteria for acceptance by consensus.
ResultsA total of 29 statements were formulated. Agreement was above 75% for all the statements, in the first round of voting. The decision was made to modify 3 of the statements, with respect to wording and content, and they underwent a second round of voting and also achieved agreement above 75%. The final statements are presented below, along with the agreement percentages, utilizing the total of the 28 voters as the denominator. In addition, the number of voters that abstained from voting, or were in disagreement, are also mentioned.
Epidemiology- 1.
The prevalence of acute pancreatitis in North America has been reported at 1 to 13 cases/100,000 children/year in school-aged children and adolescents, with a slight predominance in females. (100% agreement; 0 abstentions)
AP is an uncommon entity in children. However, in recent years a greater number of reports on its epidemiology and etiology have appeared.3–5 An annual occurrence of 1 to 13 new cases/100,000 children with AP has been calculated.1,5 In a meta-analysis that included 589 children with AP, the mean age was 9.2 ± 2.4 (SD) (range, 1 week to 21 years), with a male-to-female ratio of 1:2.6 In a study that included 55,012 children with AP, a higher frequency was reported in children > 5 years of age (mean age: 17 years), with a slight predominance in females.7 Another study, with 2,127 cases of AP, found a mean presentation age of 11.91 ± 5.38 years, observing two peaks: one between 4-5 years of age and the other in adolescents.8
- 2.
Acute pancreatitis etiology varies according to age group; in the worldwide pediatric population, the most frequent origins are biliary and idiopathic, similar to that reported in Mexico. (93% agreement; one abstention)
Numerous associated factors have been considered possible causes of AP and they vary according to age group. In the United States, a study that included 215 children showed that the most frequent causes of AP were biliary disease (32.6%) and medications (25.6%),9 whereas in another study, with 115 children, idiopathic causes (31%) and those associated with medications (23%) were predominant.10 In an Indian study on 320 children, the common causes were abdominal trauma (21%) and bile duct involvement (10%)11 and in a Chinese study on 130 children, the most common causes were biliary (31.5%), idiopathic (28.5%), and due to trauma (16.2%); metabolic problems (hypertriglyceridemia) were less frequent (9.2%) and viral infections were rare, albeit common in young children.12 In a survey conducted at Latin American centers, biliary disease, abdominal trauma, and medication intake were the most frequent etiologies.13 In Mexico, there are few case reports on AP in children and that scant information describes biliary pancreatitis and idiopathic pancreatitis as the most frequent, with a predominance in school-aged girls.14–18 AP of biliary origin is a common etiology and has been linked to an increase in childhood obesity, as an independent risk factor.19 The etiology of AP in children differs from that of adults, in whom the most frequent causes are hyperlipidemia, alcohol use, and diseases associated with multiple organ dysfunction.20
- 3.
Recurrent acute pancreatitis presents in 10 to 35% of cases, and genetic predisposition and anatomic alterations are factors to be taken into account. (Second voting round: 100% agreement; 0 abstentions)
RAP in children has been estimated at 10 to 35% of cases,18,21–23 similar to the 24 to 37% reported in Mexico.14,16,24 A relation between mutations in the PRSS1, SPINK1, CFTR, and CTRC13 genes and the presence of RAP21,23 has been shown. In contrast, a study on Mexican children with AP and RAP found the N34S mutation in the SPINK1 gene and the N29I mutation in the PRSS1 gene in 4/58 (6.8%) cases with AP and in none of the 34 cases with RAP.24 In addition to genetic factors, structural and obstructive biliopancreatic abnormalities, as well as idiopathic events, are causes of recurrence and progression to chronicity.20,22 In the INSPPIRE study cohort, pancreas divisum was found in 52 of the 359 patients (14.5%), as a risk factor for RAP. Other less frequent causes have a toxic, metabolic, or autoimmune origin.25
Clinical manifestations- 4.
Abdominal pain is the primary symptom in acute pancreatitis, but it can be absent or not clearly identified, especially in patients under 5 years of age. Vomiting and nausea are present is over half of pediatric patients with the disease. (97% agreement; one abstention)
- 5.
Comorbidities and risk factors should always be identified in the clinical approach to patients with acute pancreatitis. (100% agreement; 0 abstentions)
Abdominal pain is the cardinal symptom described in children above 4 years of age (86%), appearing in only one-third of cases under that age.26–28 Pain is most frequently located in the epigastrium, but can present in the hypochondriac region or be generalized. The classic radiating of pain into the back is present in only ≤ 10% of cases.28–31 Vomiting is the second most frequent symptom (61%), with bilious vomiting in around 20% of patients.18,28
At physical examination, children under 5 years of age tend to be hypoactive, whereas older children adopt an antalgic position. Abdominal hypersensitivity is present in 75% of cases.6,27,32 Peristalsis can be augmented, or ileus can be present.6,18,32,33 Other less frequent findings are fever, jaundice, hypocolia, hyporexia, diarrhea, ascites, abdominal mass, or pleural effusion.6,26,28,30–34 The Grey Turner’s sign, i.e., ecchymosis of the flanks, has been described, but is rare.28
- 6.
The elevation of serum amylase and/or lipase levels 3-times above the reference value is one of the diagnostic criteria of acute pancreatitis. (100% agreement; 0 abstentions)
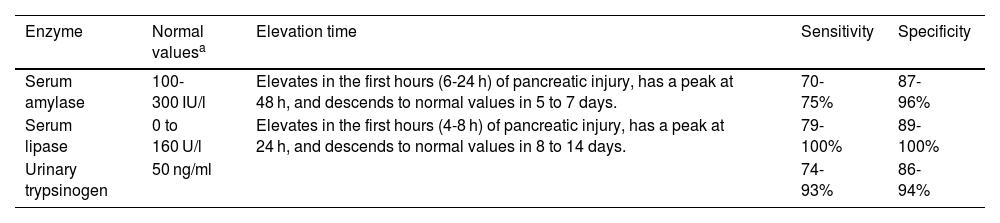
Quantifications in serum or urine can be performed, and the values vary according to the laboratory technique. Table 1 describes the normal values, elevation times, sensitivity, and specificity. Other diseases that present with elevated serum amylase or lipase levels are liver failure, kidney failure, intestinal inflammation, appendicitis, cholecystitis, peptic ulcer disease, salivary gland disorders, gynecologic diseases, abdominal trauma, diabetic ketoacidosis, and cranioencephalic trauma.35–38 Amylase can remain in normal ranges in up to 19% of patients with AP.35
Pancreatic enzymes that can be determined for diagnosing acute pancreatitis.
| Enzyme | Normal valuesa | Elevation time | Sensitivity | Specificity |
|---|---|---|---|---|
| Serum amylase | 100-300 IU/l | Elevates in the first hours (6-24 h) of pancreatic injury, has a peak at 48 h, and descends to normal values in 5 to 7 days. | 70-75% | 87-96% |
| Serum lipase | 0 to 160 U/l | Elevates in the first hours (4-8 h) of pancreatic injury, has a peak at 24 h, and descends to normal values in 8 to 14 days. | 79-100% | 89-100% |
| Urinary trypsinogen | 50 ng/ml | 74-93% | 86-94% |
During the acute episode of pancreatitis, other laboratory studies need to be performed, to identify the etiology and severity and to monitor progression: serum calcium, blood urea nitrogen, creatinine, albumin, transaminases, bilirubin, triglycerides, complete blood count, and C-reactive protein.39,40
- 7.
Abdominal ultrasound is the first choice in noninvasive imaging studies in children suspected of having acute pancreatitis or recurrent acute pancreatitis. Computed axial tomography and magnetic resonance imaging have precise indications, and their routine use is not recommended. (97% agreement; 0 abstentions)
The most useful of ultrasound findings is pancreatic duct dilation. Abdominal ultrasound (AUS) is widely available, requires no sedation, and emits no radiation. Its images can be affected by obesity and the presence of excess intestinal gas.3,41,42 Computed axial tomography (CAT) is indicated in patients with clinical deterioration or critically ill patients, but its drawback is radiation. Ideally, it should be performed ≥ 96 h after the acute episode, to prevent underestimating disease extension. The use of endovenous contrast medium is recommendable. Its capacity to detect calcifications is outstanding.38,43–45
Magnetic resonance imaging (MRI) is advantageous in cases of RAP, with increased gamma-glutamyl transferase (GGT) > 2-times higher than the normal value or the presence of cholestasis identified by AUS, with no evidence of bile duct obstruction. It detects diseases, such as pancreas divisum, or pancreatobiliary junction abnormalities. The use of secretin improves visualization of the bile ducts and the pancreatic duct.38,41,46,47
Endoscopy in the diagnosis and treatment of acute pancreatitis- 8.
Esophagogastroduodenoscopy is not a first-choice study in acute pancreatitis, and its use should be individualized. (97% agreement; 0 abstentions)
Inflammatory disease of the duodenal mucosa, such as celiac disease or Crohn’s disease, increases the risk for developing AP. Upper gastrointestinal endoscopy should be elective, once the acute episode is resolved, as part of the etiologic approach, or in patients suspected of presenting with duodenal obstruction (tumor, stricture). Its usefulness should be weighed in each case, and considered in patients with comorbidities, such as hematemesis or persistent vomiting.38
- 9.
Endoscopic retrograde cholangiopancreatography in pediatric patients should be performed for therapeutic purposes. (100% agreement; 0 abstentions)
The current performance of noninvasive studies, such as contrasted CAT or cholangioresonance, has displaced diagnostic endoscopic retrograde cholangiopancreatography (ERCP) in pediatric patients. ERCP is recommended in patients with AP suspected of presenting with biliary obstruction (dilated choledochus, obstructive jaundice, signs of cholangitis) in the early phase, and for treatment of complications, such as pancreatic fistula or pseudocyst drainage in the late phase.38,48,49
- 10.
Biliary pancreatitis secondary to choledocholithiasis is the primary indication for endoscopic retrograde cholangiopancreatography, to decompress the bile ducts and pancreatic duct. (100% agreement; 0 abstentions)
There are no specific recommendations in pediatrics, regarding the ideal time to carry out ERCP. In a meta-analysis on adults, its performance is recommended within the first 48 h from symptom onset in the presence of obstructive jaundice or cholangitis and leaving it as an elective procedure in patients with uncomplicated choledocholithiasis. Freeing the bile duct improves drainage of the pancreatic duct, reducing intraductal pressure. In Mexico, only therapeutic videoscopes measuring 11 mm in diameter are available, thus ERCP can only be performed on patients weighing more than 10 kg. If there are no surgeons experienced in therapeutic ERCP, the patient should be referred to a specialized center or surgical treatment should be considered.48,50
- 11.
A pancreatic pseudocyst can develop in acute pancreatitis; when it is symptomatic or causes complications, such as gastric, intestinal, or bile duct obstruction or infection, it may require endoscopic or surgical intervention. (Second voting round: 97% agreement; one abstention)
Pancreatic pseudocysts develop in 10-23% of pediatric patients with AP and can cause abdominal pain, vomiting, oral intolerance due to gastric compression, intestinal obstruction, or biliary obstruction. Derived from experience in adults, drainage could be considered, when the pseudocyst is larger than 6 cm and persists for more than 6 weeks. The content can become infected, and require endoscopic, percutaneous, radiologic, or surgical drainage. Pseudocysts that compress the gastric or duodenal walls, can be drained through transmural endoscopy, with plastic stent placement. In patients in whom the main pancreatic duct communicates with the pancreatic pseudocyst, ERCP enables the placement of a transpapillary stent in the duodenum, re-establishing duct drainage, thus preventing leakage into the pseudocyst.51–56
- 12.
Endoscopic ultrasound is ideal for draining pseudocysts that do not produce compression; ultrasound visualization enables vascular structures to be avoided, defines the characteristics of the collection (fluid-filled, solid), and determines the best access site. (90% agreement; one abstention)
An estimated 42-48% of pancreatic pseudocysts do not produce compression on the digestive tract wall and cannot be safely and efficaciously drained by means of conventional endoscopy. Transmural puncture, guidewire placement, balloon dilation, and the placement of one or several double-pigtail plastic stents to facilitate drainage can be carried out through endoscopic ultrasound, as well as the placement of fully covered self-expandable metallic stents > 10 mm, if there is solid necrotic material. There is a reported success rate of 91% and pseudocyst resolution in 93%.53
- 13.
In traumatic acute pancreatitis, with a fracture in the main pancreatic duct, endoscopic retrograde cholangiopancreatography enables the extension of the damage to be located and the injury of the duct to be treated. (Second voting round: 93% agreement; one abstention)
In children, pancreatic trauma presents in < 2% of cases of abdominal trauma and can include peripancreatic bleeding/hematoma, pancreatic laceration, and complete transection of the gland, associated with different degrees of ductal injury.57 Through ERCP, the anatomic integrity of the main pancreatic duct can be evaluated (ductal damage is the main predictor of morbidity and mortality), with the therapeutic possibility of stent placement, in case of a leak. The majority of cases do not require surgical intervention, and if needed, treatment should be individualized, based on the exact grade of the lesion. Surgery is reserved for grades III-V or in the presence of signs of peritoneal irritation or hemodynamic instability.38,58
- 14.
Pancreatitis following endoscopic retrograde cholangiopancreatography has a prevalence of 9-17%, in pediatrics. Preventive measures, such as rectal indomethacin, ketorolac, and hyperhydration, are recommended. (93% agreement; 2 abstentions)
Intrarectal indomethacin in adults has been shown to reduce the incidence of pancreatitis following ERCP. In pediatrics, its use is restricted to adolescents because it cannot be dosed (50 and 100 mg suppositories).59–62 In small children that are at high risk, there is evidence of success with the use of ketorolac IV at 0.5 mg/kg/dose (incidence of 11% vs. 25%, p = 0.035), but not with other nonsteroidal anti-inflammatory drugs.63,64 Hyperhydration with Ringer’s lactate solution, at 3 ml/kg/h during the procedure, and then at a single 20 ml/kg dose, has been shown to be useful for preventing the disease (incidence of 0% vs. 17%, p = 0.016).65,66
Medical treatment- 15.
Initial intravenous treatment with glucose and crystalloid solutions is recommended in children with acute pancreatitis, as well as hyperhydration 1.5 to 2-times above the recommended daily intake. There is currently insufficient evidence for recommending Ringer’s lactate solution over saline solution in children, but it is recommended in adults. (90% agreement; 2 abstentions)
Initial hydration with glucose and crystalloid solutions is essential in the management of AP in children.38,67 In a systematic review on adults with AP, the use of Ringer’s lactate solution, compared with saline solution, was found to have a greater anti-inflammatory effect, improve clinical results, and reduce the presentation of adverse events.68 There are no clinically controlled trials on the type of intravenous solutions to be used in AP in pediatrics. In a retrospective multicenter pediatric study, the use of Ringer’s lactate solution was associated with reduced admission costs and days of hospital stay.69
- 16.
Non-narcotic analgesics, such as paracetamol, metamizole, ketorolac, or ibuprofen, can be first-line treatment for pain in pediatric acute pancreatitis. Narcotics can be used in cases of intense and uncontrollable pain. (100% agreement; 0 abstentions)
There are few controlled studies on analgesic management, regarding AP in pediatrics, and so the majority of scientific evidence is extrapolated from studies on adults and recommendations for that age group.38
Analgesia in AP involves a wide range of drugs, that should be used in an individualized manner, according to the presentation of the clinical symptoms.38,67–70
The safety of opioid use, especially morphine, is a subject of debate, given that the risk for increased sphincter of Oddi tone, which can exacerbate symptoms, is attributed to opioids. Nevertheless, that has not been demonstrated through manometric studies of the sphincter of Oddi, with adequate methodology.71,72 In contrast, different clinical trials and a meta-analysis have shown the safety and analgesic efficacy of buprenorphine, pethidine (meperidine), pentazocine, fentanyl, and morphine.71,73,74
- 17.
The use of orogastric or nasogastric tubes is not recommended for decompressing the stomach. Their use is limited to cases of metabolic ileus and uncontrollable vomiting. (100% agreement; 0 abstentions)
The use of feeding tubes was previously thought to be indicated for reducing pancreatic enzyme activity, and in that manner, recover function more quickly, but that practice is currently considered obsolete. Enteral diet should be started as soon as possible, whether orally or with feeding tubes.30,75–77
- 18.
No evidence has been published that justifies the routine use of proton pump inhibitors in patients with acute pancreatitis. (100% agreement; 0 abstentions)
Some authors recommend the prophylactic use of proton pump inhibitors, in cases of severe AP, with the risk for developing stress ulcers, but they are only indicated when there is peptic ulcer or duodenal ulcer, gastroesophageal reflux disease, or upper gastrointestinal bleeding.78–82
- 19.
Probiotic use in pediatrics is not recommended due to a lack of evidence in children and controversial studies on adults. (100% agreement; 0 abstentions)
Probiotics have been considered promising, with respect to improving the dysbiosis involved in AP and its severity. There are reports describing the potential benefit of fewer days of hospital stay and reduced symptom severity, whereas others describe an increase in mortality (the PROPATRIA study). The lack of controlled and unified studies, with respect to strains and doses to be utilized, makes it difficult to emit a recommendation in favor of their use.67,83–87
- 20.
Antioxidants are not recommended in the medical treatment of children with acute pancreatitis. (100% agreement; 0 abstentions)
Oxidative stress participates in the pathophysiology of AP, through the formation of oxygen-free radicals that damage the cells of the pancreas. The administration of antioxidant nutrients, such as retinol, ascorbic acid, and tocopherol, and inorganic nutrients, such as selenium, in patients with AP, has shown controversial results. There is no scientific evidence related to antioxidants in AP in pediatric patients.38,88–91
- 21.
The prophylactic use of antibiotics is not indicated in acute pancreatitis, regardless of its severity. Antibiotics that penetrate necrotic tissue are indicated in the management of pancreatic necrosis and infected extrapancreatic collections, and in patients with necrotizing pancreatitis that do not show clinical improvement and present with signs, symptoms, and laboratory data suggestive of infection. (100% agreement; 0 abstentions)
Severe AP can be associated with bacterial translocation, bacteremia, secondary pancreatic infection, and sepsis. The prophylactic use of antibiotics is controversial and current evidence shows they are only indicated in suspected or confirmed cases of pancreatic or extrapancreatic infection. The published evidence on antibiotic treatment in AP is in adults.38,92–95
Nutritional treatment- 22.
Oral nutrition or enteral nutrition in mild acute pancreatitis should be started early (the first 72 h of hospital admission) with a normal diet, progressing to tolerance. (100% agreement; 0 abstentions)
Oral nutrition or enteral nutrition (EN) is an active therapeutic intervention that improves the progression of patients with AP and is associated with lower costs and lower morbidity and mortality rates.38,67,96–98 The mechanisms that sustain early feeding or EN in AP are the modulation in the systemic inflammatory response and the decrease in cytokine secretion, as well as reduced intestinal villi atrophy, luminal stasis, and intestinal permeability; all of those factors result in a lower risk for bacterial translocation and sepsis.38 Early initiation does not affect the exocrine function of the pancreas.38,96,97
The resumption of oral nutrition or EN has been demonstrated to be safe in mild AP, when performed within the first 72 h of hospital admission, in the presence of adequate intestinal conditions, and progressing, according to tolerance, until achieving the total energy requirements, even in the presence of signs of systemic inflammation. Serum amylase and lipase values do not need to normalize, nor does pain have to cease completely, even when analgesia is continued.38,67,96,97
In a small cohort of 38 children with mild AP, oral nutrition or EN were well tolerated and were not associated with abdominal pain.99 In another study by the same group, those authors found that early feeding or EN in mild AP was associated with shorter hospital stay, fewer admissions to the intensive care unit, and a lower frequency of severe AP.100 In a series of 51 episodes of AP in 32 Mexican children, early EN, started in 62.7% of the patients, was associated with shorter hospital stay.15
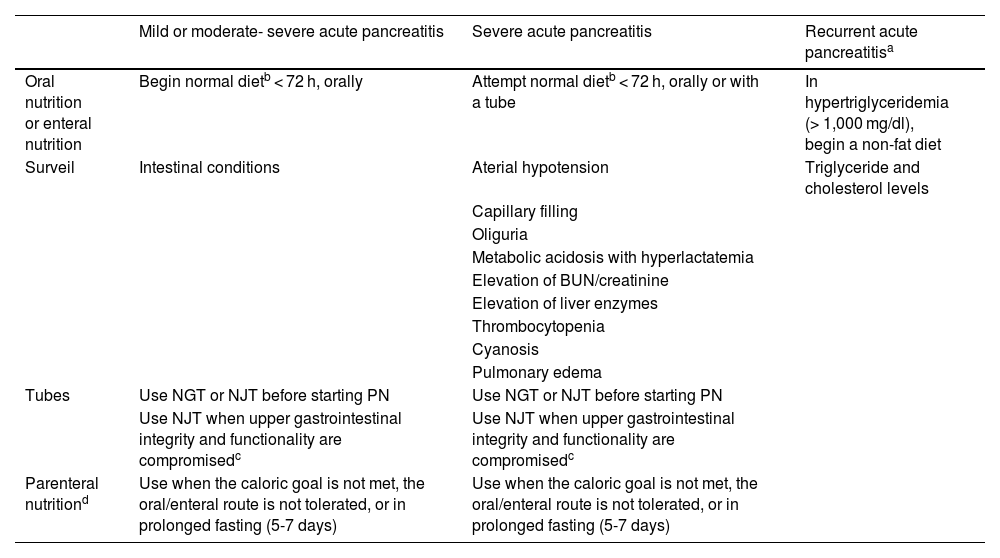
There is insufficient evidence that nutrition through a nasojejunal tube is more beneficial than nutrition through a nasogastric tube, in pediatric patients with moderate-to-severe AP.101 The recommendations from the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) in 2019 indicate nasojejunal tube use in cases in which the integrity and functionality of the upper gastrointestinal tract are compromised (Table 2).102
Nutritional treatment and clinical surveillance in acute pancreatitis and recurrent acute pancreatitis.
| Mild or moderate- severe acute pancreatitis | Severe acute pancreatitis | Recurrent acute pancreatitisa | |
|---|---|---|---|
| Oral nutrition or enteral nutrition | Begin normal dietb < 72 h, orally | Attempt normal dietb < 72 h, orally or with a tube | In hypertriglyceridemia (> 1,000 mg/dl), begin a non-fat diet |
| Surveil | Intestinal conditions | Aterial hypotension | Triglyceride and cholesterol levels |
| Capillary filling | |||
| Oliguria | |||
| Metabolic acidosis with hyperlactatemia | |||
| Elevation of BUN/creatinine | |||
| Elevation of liver enzymes | |||
| Thrombocytopenia | |||
| Cyanosis | |||
| Pulmonary edema | |||
| Tubes | Use NGT or NJT before starting PN | Use NGT or NJT before starting PN | |
| Use NJT when upper gastrointestinal integrity and functionality are compromisedc | Use NJT when upper gastrointestinal integrity and functionality are compromisedc | ||
| Parenteral nutritiond | Use when the caloric goal is not met, the oral/enteral route is not tolerated, or in prolonged fasting (5-7 days) | Use when the caloric goal is not met, the oral/enteral route is not tolerated, or in prolonged fasting (5-7 days) |
BUN: blood urea nitrogen; NGT: nasogastric tube; NJT: nasojejunal tube; PN: parenteral nutrition.
There are not enough studies on the pediatric population to emit an absolute recommendation, with respect to different types of liquids that should be used to start EN (clear liquids, whole milk, or polymeric, elemental or semi-elemental formulas).97,103
There is sufficient evidence on the benefit of starting oral nutrition or EN and it should be done as soon as possible.38
- 23.
In severe acute pancreatitis, oral nutrition or enteral nutrition should be attempted within the first 72 h of hospital admission, once the patient is stabilized. (97% agreement; one abstention)
There is evidence in the adult population with severe AP on the safety and efficacy of oral nutrition or EN, within the first 48 h of hospital admission, with a decrease in gastrointestinal symptoms, local complications, systemic infections, need for surgery, multiple organ failure, mortality, and hospital stay.104–108 Hospital stay was shorter even in the presence of abdominal pain and elevated pancreatic enzymes.108 Pediatric studies have shown that starting early oral nutrition or EN reduced complications, hospital stay, and mortality in patients admitted to the intensive care unit.97,101 Superiority over parenteral nutrition (PN) for reducing mortality, infectious and surgical complications, and organ failure, has also been shown in adults.109,110 Nevertheless, there is still doubt about starting early EN as a treatment measure. A Canadian study on 223 children reported that EN was usually started late in moderate-severe or severe AP (9 days, IQR 5-15 days), compared with mild AP (3 days, IQR 1-3 days), respectively (p < 0.0001).111
The purpose of oral nutrition or EN in severe AP (confirmed or predicted) is to maintain the function of the intestinal barrier, and it should be attempted early, except when there are direct contraindications (ileus, complex fistula, abdominal compartment syndrome) or some other contraindication for resuming oral/enteral feeding, once the patient’s hemodynamic and metabolic stability are achieved (Table 2).97,112
- 24.
There is no scientific evidence supporting the indication of a low-fat diet over a normal diet in episodes of acute pancreatitis. (97% agreement; one abstention)
A completely solid diet with no fat restrictions is safe and well tolerated in patients that can be fed through the oral/enteral route because fat ingestion does not affect progression in mild-moderate AP.97,113 A study that included 33 children from 2 to 18 years of age, with mild-moderate AP, divided them into 2 groups: 1) fasting with intravenous liquids and then a low-fat diet, and 2) feeding with no restrictions within 24 h after symptom onset. The results showed that the early start of a diet with no fat restrictions was safe and made no difference, with respect to the time of discharge, readmission rates, and decrease in lipase levels, compared with the subgroup of initial fasting, followed by a low-fat diet.98 Another study showed that length of hospital stay and lipase values, in children hospitalized with mild AP with a mean fat intake < 0.5 g/kg/day and > 1 g/kg/day (low 0-0.5 g/kg/day, normal 0.5-1 g/kg/day, and high > 1 g/kg/day), were not significantly impacted. Greater fat intake was associated with significantly lower daily pain severity scores (p < 0.001).99,113
The treatment of hyperglyceridemia-associated RAP requires a low-fat diet to manage that metabolic disorder.103 However, according to the latest (2018) consensus of the ESPGHAN, the term “low-fat” is not well defined in the majority of reports. Those guidelines refer to a pediatric diet that is “normal in fat” as one that contains 30-40% of lipids for children 1-3 years of age and 25-35% for children 4-18 years of age.97 The percentage of fat during the first 6 months is recommended at 40-60%, to then gradually decrease to 35% at 2 years of age and to 25-35% after 2 years of age.114
Until there is evidence against a normal fat diet, not restricting fat in AP is recommended.113
- 25.
Oral/enteral nutrition is preferred over parenteral nutrition, alone or combined. (100% agreement; 0 abstentions)
In a meta-analysis on pediatric patients, a mortality rate for EN of 7% was identified, versus 20.7% for total PN (TPN). Likewise, in adults, the early start of feeding or EN showed a decrease in mortality of 50%, compared with the exclusive use of TPN.115,116
TPN should be reserved for cases in which the oral/enteral route is not tolerated and considered when EN is not possible over a prolonged period (5-7 days). At present, there is no solid evidence on the use of PN combined with oral nutrition or EN, but that intervention is recommended, mainly in patients that do not meet their caloric goal or when there are direct contraindications for the enteral route.97,113,116 In cases of oral/enteral route contraindication, the recommendations on TPN in the current guidelines for the critically ill pediatric patient should be followed (Table 3).117–121
Recommendations for the use of parenteral nutrition in the patient with acute pancreatitis.
| Recommendations | |
|---|---|
| Energy | Indirect calorimetry should be used. In its absence, prediction formulas (WHO/Schofield) can be useful, always considering the nutritional progression of the patient for establishing energy goals.117,118 |
| Protein | Ensure a minimum of 1.5 g/kg in the critical stage to prevent a negative protein balance and muscle mass loss.117,118 |
| Lipids | Limiting to 3 g/kg/day is recommended. The fact that lipoprotein lipase activity is influenced by nutritional status, hypoalbuminemia, metabolic acidosis, dyslipidemia, and can be reduced in a state of stress and catabolism, should be considered; if the lipid infusion rate exceeds the lipid transport and clearance rate, triglycerides will increase.119 |
| Glucose | In the management of the critical patient, maintaining glucose homeostasis is essential, making it necessary to follow the guidelines established by weight (mg/kg/min or g/kg/day). Hyperglycemia (> 145 mg/dl) is associated with an increase in morbidity and mortality in the critically ill patient.120 |
| Micronutrients | Ensure the requirements for each age group. Parenteral nutrition should not be utilized to correct serum electrolytes.117,118 |
| Additional supplements | There is no evidence supporting the routine use of L-carnitine, with respect to the lipid profile, weight gain, or weight loss during hospital stay.121 |
- 26.
Surgical treatment in necrotic acute pancreatitis is uncommon. (97% agreement; one abstention)
Treatment should be carried out in “ascending steps”. The indication for surgery is made in patients with poor response to medical treatment, progressive deterioration, multiple organ dysfunction and/or infected necrotic collections, starting with the less invasive treatments.122 Percutaneous, endoscopic, and surgical interventions are among the treatment modalities. Percutaneous drainage reduces the need for surgical treatment in up to 35% of cases in adults.122 Surgical treatment is recommended between the third and fourth week of progression.38,123,124 Necrosectomy is performed using the organ-sparing technique with blunt dissection, to minimize the risk for bleeding, fistula, or removal of living tissue.125 The surgical techniques include the laparoscopic approach, minimally invasive retroperitoneal necrosectomy, and retroperitoneal necrosectomy. In adults, no technique has been shown to be better than another.23,125 In addition, the patient with necrotic AP may require surgical evaluation due to intestinal, gastric, or pleural fistulas, bleeding, and compartment syndrome.126
- 27.
A pancreatic pseudocyst is a complication that rarely merits surgical treatment. (93% agreement; one abstention)
The large majority of pseudocysts resolve spontaneously within the third and fourth month. When drainage is required, the treatment of choice is endoscopic or percutaneous. However, surgical treatment may be indicated when there is complex narrowing of the pancreatic duct, pseudocyst in the tail of the pancreas contiguous with the spleen, severe compression of the main bile duct and duodenum, multiple or recurrent pseudocysts, vascular complications not resolved through angiography, and failed endoscopic or percutaneous treatment.127–129 Treatment consists of creating an anastomosis between the gastrointestinal tract and the cyst. Different techniques that can be performed through laparoscopy or as open interventions have been described: cystogastrostomy, cystoduodenostomy, cystojejunostomy, central pancreatectomy, and pancreatojejunostomy.130–133 In a systematic review spanning 10 years, laparoscopic drainage was successful in 98.3% of patients, 2.5% had recurrence, and < 2% had complications.132 In a meta-analysis conducted on adults, the laparoscopic approach was associated with less bleeding and shorter procedure duration, compared with the open approach.133
- 28.
There is no laboratory marker or validated scale that can accurately predict the patients at risk for developing severe pancreatitis in pediatrics. (100% agreement; 0 abstentions)
Debanto published the first pediatric score for determining severity risk in pancreatitis. Coffey et al. found that an increase in the baseline value of serum lipase > 7-times the value at admission, increases the risk of severe pancreatitis (OR 7.1, 95% CI 2.5-20.5), NPV 89%, and PPV 46%.37 The main drawback of those studies is that they were conducted without a severe AP criterion in pediatric patients.2
Recently, a study found that serum levels of blood urea nitrogen > 13 mg/dl and albumin < 3.6 g/dl had 71% sensitivity, 79% specificity, 60% PPV, and 86% NPV for determining severe AP.134 The same group found that the elevation of serum levels of IL-6, monocyte chemotactic protein (MCP-1), and/or C-reactive protein, increased the risk for severe AP. Those studies, in concordance with the criteria proposed by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, are promising and open the possibility of conducting prospective studies for corroborating those findings.135
- 29.
In patients with a first event of mild acute pancreatitis that have biliary lithiasis, cholecystectomy performed during the same hospitalization is suggested. (97% agreement; one abstention)
There is scientific evidence that cholecystectomy as treatment for gallstones in biliary pancreatitis can be performed safely and that it prevents the recurrence of pancreatitis in the pediatric population, compared with delayed cholecystectomy (0-4% vs. 22-36%).136–139 Likewise, a decrease in the risk for readmission due to pancreatitis has been reported in up to 57% of patients, when the procedure is performed within the first 2 weeks of the first episode of the disease.140
With respect to surgical complications (bleeding, wound infection, bile duct injury, and prolonged hospital stay), there is also greater evidence in controlled studies and meta-analyses on adults that shows no difference between the groups that underwent early cholecystectomy (same-admission cholecystectomy, and even on the same day), compared with the groups that underwent delayed cholecystectomy, with a difference in risk of –0.0016, 95% CI (–0.04-0.04) and OR 0.78, 0.38 to 1.62).141–145
Ethical considerationsBecause this document is a consensus, based on the best published scientific evidence, and is not a research study on patients, requesting informed consent from patients to receive treatment to participate in the study was not required. No experiments were conducted on animals and/or humans.
Given the descriptive nature of the document and the fact that it is a position document of the association, authorization by an ethics committee was not required.
The authors declare that this article contains no personal information that could identify patients.
Financial disclosureNone.
Conflict of interestAll the authors declare they have no conflict of interest regarding the development of this consensus.
Please cite this article as: Vázquez-Frias R, Rivera-Suazo Y, Aguayo-Elorriaga AK, Alfaro-Bolaños JE, Argüello-Arévalo GA, Cadena-León JF et al. Consenso de la Asociación Mexicana de Gastroenterología sobre el diagnóstico y tratamiento de pancreatitis aguda en niñas, niños y adolescentes. Rev Gastroenterol Méx. 2023;88:267–281.