Crohn’s disease can affect the stomach, small intestine, large intestine and/or perianal region. It has a recurrent disease course, and the therapeutic regimen tends to be modified.1,2
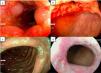
A 30-year-old woman had symptom onset with diarrhea, abdominal pain, and perianal pain. She presented with what was considered a moderate disease flare affecting the ileum, colon, and perianal region, for which 390 mg of ustekinumab was administered intravenously, followed by 90 mg subcutaneously every eight weeks, as the maintenance regimen. The patient was in clinical and endoscopic remission for one year. She then presented with a moderate disease flare-up (the hospital was not able to measure serum levels of ustekinumab or antibodies to the drug, nor was another biologic agent available), and so ustekinumab re-induction was administered and maintenance therapy was shortened to every four weeks. Clinical remission was achieved for six months, after which the patient presented with a moderate disease flare of 225 points on the Crohn’s disease activity index (CDAI). Ulcers were present in the terminal ileum (Fig. 1a), the ileocecal valve (Fig. 1b), the transverse colon (Fig. 1c), and in the anal canal (Fig. 1d), with a Crohn’s disease endoscopic index of severity (CDEIS) score of 24. As a result, the medication was switched to 45 mg/day of upadacitinib (the only medication different from ustekinumab available at the hospital at that time). The patient is currently in her sixth month of treatment, with clinical (CDAI score of 29) and endoscopic (CDEIS score of 2) remission.
Age under 40 years, moderate-to-severe disease presentation, and perianal involvement are poor prognosis factors, making the use of biologic agents necessary for achieving remission.3,4 In a systematic review of 38 studies on ustekinumab in patients with Crohn’s disease, 60% of patients achieved clinical response and 34% achieved remission with induction therapy; at one year of said therapy, 42% maintained response and 31% were still in remission.5 Upadacitinib has the capacity to reduce proinflammatory cytokine production.6 Different clinical trials have shown that patients with moderate-to-severe Crohn’s disease respond favorably to induction and maintenance therapy with upadacitinib, compared with placebo.7–9 In different clinical trials, patients with perianal disease are excluded because they are more difficult to treat. Clinical response to a second biologic agent also tends to be less efficacious. Thus, a patient that responds adequately is a subject of interest. The aim of this study is to present patients with perianal involvement an alternative treatment.
In conclusion, upadacitinib was shown to be effective as rescue therapy in a young female patient receiving ustekinumab, whose disease involved the ileum, colon, and perianal region.
Ethical considerationsThe authors declare that no experiments on humans were performed in this research. We observed our work center’s protocols for obtaining patient databases and preserving patient anonymity (thus informed consent was not requested). This study meets the current bioethical research regulations.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.