Inflammatory bowel disease (IBD) in the pediatric population is increasing, reaching up to 30% of the total IBD population.1 In Latin America, there is scant information on the epidemiology of the disease in pediatrics, but a study published in 2021 confirmed an increase in the number of cases.2
Different therapeutic strategies have been approved for the treatment of IBD,3 but the pivotal studies supporting their use have been conducted on the adult population and their results tend to be extrapolated to the pediatric population. Regarding non-response to anti-tumor necrosis factor (anti-TNF) inhibitors, medications with other mechanisms of action that are not approved for pediatric use are prescribed “off-label” as rescue therapy in cases of refractoriness. One such medication is the small molecule Janus kinase (JAK) inhibitors.4–8 To the best of our knowledge, there are no published case reports in Latin America on the use of upadacitinib, a selective JAK1 inhibitor, in the pediatric population with Crohn’s disease (CD).
A 15-year-old female patient with an unremarkable past medical history presented with daily pasty stools with fresh blood and peripheral articular pain, starting in December 2023. Laboratory workup showed hemoglobin 9 g/dl, normal C-reactive protein, albumin 3.7 g/dl, fecal calprotectin (FC) 441 µg/g, and negative microbiologic study. Colonoscopy was performed, revealing a conserved terminal ileum, as well as erosions and 2 mm ulcers interspersed with unaffected mucosal areas. Treatment was started with 40 mg prednisone for 8 weeks and 2 g of oral mesalazine daily. However, after suspending the corticosteroid and maintaining mesalazine, the patient once again presented with bloody diarrhea, weighted pediatric Crohn’s disease index (wPCDAI) 52.5, and FC 1120 µg/g. She was evaluated at our center. Colonoscopy revealed ulcers in the terminal ileum and colon consistent with CD with moderate inflammatory activity (simple endoscopic score-Crohn’s disease [SES-CD] 11). Magnetic resonance enterography of the abdomen and pelvis confirmed inflammatory involvement of the terminal ileum and segmental involvement at the level of the descending colon-sigmoid colon. CD was diagnosed and described as A1b, L3, B1, G0, using the Paris classification, and treatment with adalimumab was started. However, at 16 weeks, the bloody diarrhea persisted, and the patient had FC levels of 144 ug/g and pre-dose adalimumab plasma levels of 24.5 ug/mL.
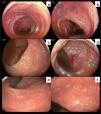
A new colonoscopy confirmed ileocolonic CD with severe inflammatory activity (SES-CD 30) (Fig. 1a–d). Due to primary non-response to adalimumab, treatment with induction upadacitinib was started (45 mg daily, taken orally for 12 weeks). The patient’s progression was favorable, achieving symptom remission, wPCDAI 0, and endoscopic response, with the presence of aphthous ulcers exclusively in the last 2 cm of the distal rectum, SES-CD 3 (Fig.1e and f). Rectal biopsies only showed lymphoplasmacytic infiltration, with no alterations in the glandular architecture. Thus, the decision was made to continue treatment with upadacitinib, employing it as maintenance therapy (30 mg day, taken orally). At present the patient is asymptomatic and has not presented with adverse events due to the drug.
In the pediatric population with IBD, similar to that described in adults, primary non-response has been observed in 25–30% of cases, as well as a secondary loss of response in up to 60% of cases utilizing anti-TNF therapy.9,10 In that context, studies have been published on the effectiveness of other therapeutic strategies, including the use of vedolizumab and ustekinumab, biologics with a different mechanism of action,4 or the Janus-kinase (JAK) small molecule drugs.5–8
In general, the choice of drug should be personalized, considering patient profile, disease characteristics, the desire for pregnancy, access possibilities, administration route, and importantly, patient opinion.6
Our case, like those described in other reports,7,8 showed that upadacitinib is a therapeutic option in pediatric and adult populations that have experienced previous non-response to anti-TNF inhibitors. For our patient, the available options were infliximab, ustekinumab, vedolizumab, or upadacitinib. The selection of the medication was discussed by the patient’s family and due to more favorable coverage, times, and response possibilities, the decision was made to start upadacitinib. Our patient achieved symptom remission after drug induction, as well as endoscopic response.
Regarding upadacitinib safety, even though possible adverse events are described, they have been reported as manageable. However, long-term studies and close monitoring are needed for its validation.7,8 Our patient has not presented with any adverse events during the treatment period.
The present case demonstrates that upadacitinib use can induce clinical and endoscopic remission in pediatric CD refractory to anti-TNF therapy, resulting in successful rescue therapy. Even though it is an individual report, it provides encouraging evidence in favor of formally studying the safety and efficacy of JAK inhibitors in the pediatric population with refractory IBD.
Ethical disclosuresThe authors declare that no experiments were conducted on humans or animals for the present study, that they have followed the protocols of their work center on the publication of patient data, and that they have preserved patient anonymity at all times. Informed consent was not required for the publication of the present case because the article contains no personal data that could identify the patient. This work meets the current bioethical research regulations.
Financial disclosureNo financial support was received in relation to this article.
The authors declare that there is no conflict of interest.