Extramedullary plasmacytoma (EMP) is a proliferation of plasma cells outside of the bone marrow that may develop either independently or associated with multiple myeloma.1 Most EMPs affect the upper respiratory tract and the head and neck region. Location in the digestive tract is unusual and occurs in less than 5% of cases. Gastrointestinal involvement is associated with various symptoms, such as abdominal pain, weight loss, and gastrointestinal bleeding.2
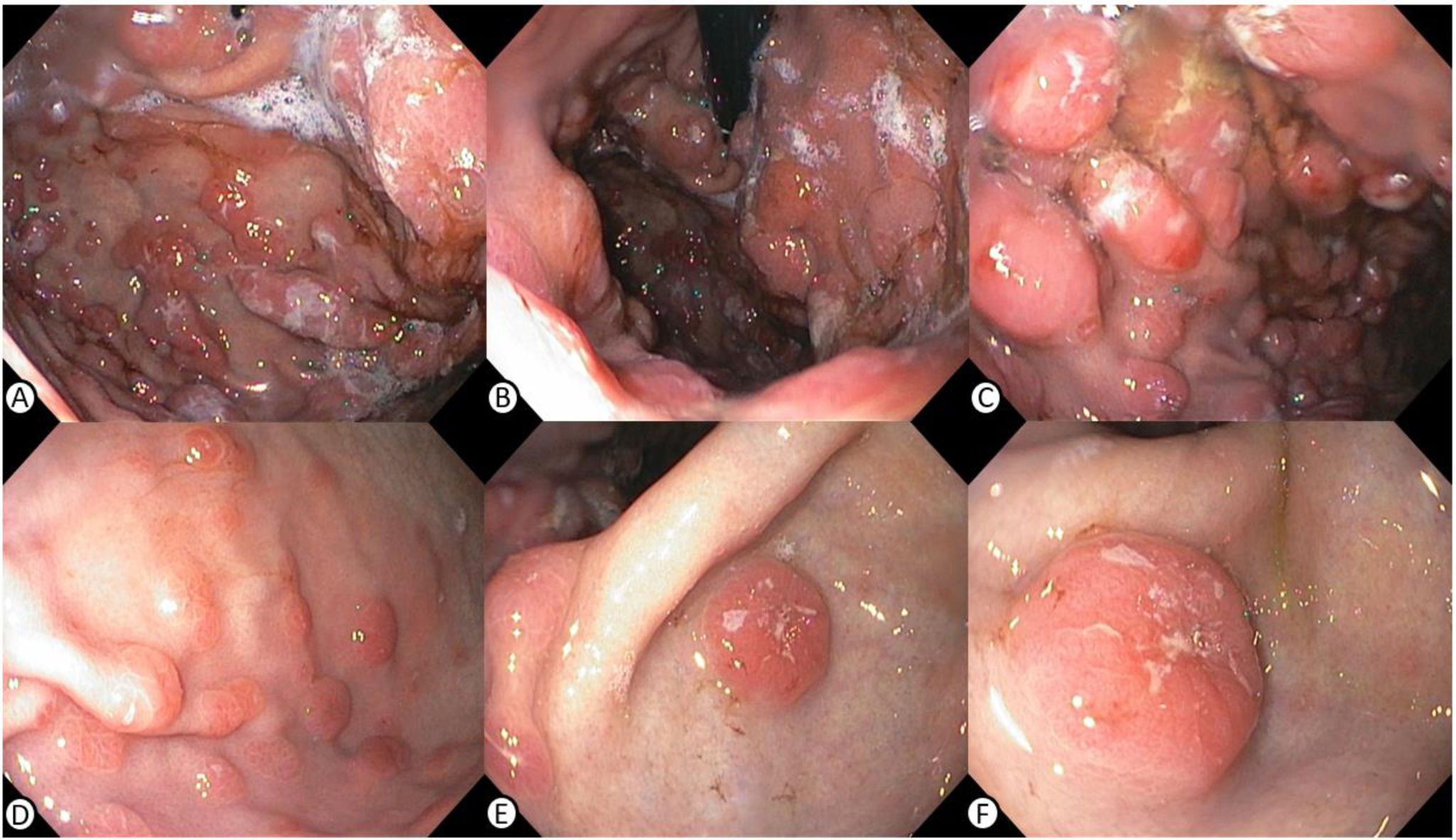
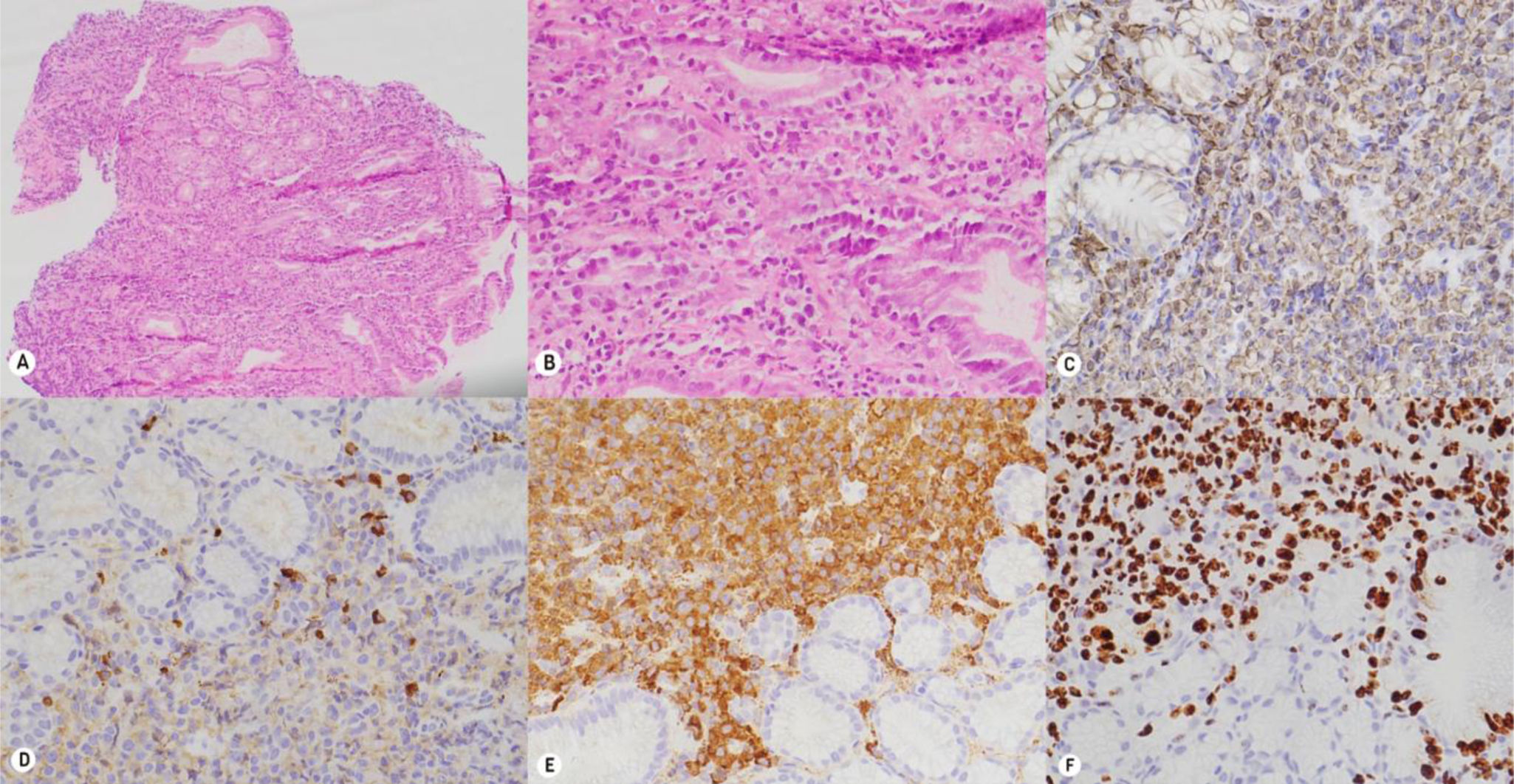
A 38-year-old man diagnosed with human immunodeficiency virus (HIV), with poor adherence to antiretroviral therapy and an advanced immunosuppression status (CD4 396 cell/µl and a viral load of 319,269 copies/mL), sought medical attention for chest pain, headache, fever, and tarry stools of 20-day progression, added to constitutional symptoms resulting from involuntary weight loss of 6 kg in the past month. During hospitalization, lytic lesions in the chest, skull, and cervical spine were documented, along with focal lesions in the liver and gastric wall thickening in cross-sectional images of the abdomen. Upper gastrointestinal endoscopy revealed raised erythematous, lobulated lesions with central depression located in the gastric fundus, body, and antrum, findings highly suggestive of neoplastic involvement (Fig. 1). The antral biopsies showed a plasmacytoid cell infiltrate, with positivity for CD138, MUM 1, Lambda, CD3, CD20, C-MYC (40%), and Ki67 (80%) (Fig. 2), supporting the suspected lambda plasma cell tumor involvement, possibly presenting as gastric EMP. Given the unusual presentation of upper gastrointestinal involvement, associated with the immunosuppression status and Epstein-Barr virus-encoded small RNA in situ hybridization (EBER-ISH) positivity < 15%, the possibility of plasmablastic lymphoma was posited as the differential diagnosis. Management with the dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP) protocol was started and at outpatient follow-up, the response to chemotherapy and progression of the gastrointestinal lesions were evaluated.
A and B) Multiple raised erythematous, lobulated lesions with central depression located in the gastric fundus, viewed through retroflexion. C and D) Lesions running through the greater curvature of the gastric body. E and F) Raised erythematous and umbilicated lesion located on the anterior wall of the antrum, a sample of which was obtained for histopathology.
Histopathology and immunohistochemistry. A and B) Hematoxylin and eosin staining. The slices show the gastric mucosa with expansion of the lamina propria, due to the dense lymphoplasmacytic infiltrate, which infiltrates the superficial and glandular epithelium. C–F) The inflammatory infiltrate is positive for CD138; KI67 shows restriction for the lambda chain.
Plasmacytomas are rare entities characterized by the localized growth of monoclonal plasma cells. They have two main forms: solitary plasmacytoma of bone and EMP, the latter of which accounts for 3–5% of plasma cell tumors and has a 10–20% risk of progressing to multiple myeloma.1
Gastrointestinal plasmacytoma is unusual, representing less than 5% of EMPs, and may be under-diagnosed. There are no available studies on a Mexican population that document its incidence or mortality rate, highlighting limitations in the local epidemiologic knowledge of the entity. It generally affects the small bowel, followed by the esophagus, stomach, and colon. Middle-aged men (55 years of age) predominate, and symptoms are related to location and include weight loss, nausea, vomiting, malabsorption, obstruction, and bleeding.
The clinical picture of gastrointestinal EMPs is nonspecific, supporting the performance of additional studies, such as cross-sectional images of the abdomen, upper and lower endoscopy, and biopsies with immunohistochemistry testing. Together, those tools aid in making the definitive diagnosis. Endoscopic lesions vary and include ulcerated masses, thickened folds, polyps, and diffuse lesions similar to linitis plastica. Histologically, there is infiltration of atypical plasma cells, with eccentric nuclei and a “cartwheel” chromatin pattern. Immunohistochemistry identifies CD138, MUM1, and light chain kappa or lambda markers.1
In patients with HIV, plasma cell disorders, such as multiple myeloma, are frequent and they tend to present as EMP, with rapid progression and poor prognosis. Chronic antigenic stimulation and immunodeficiency are thought to favor its development, although the precise mechanism is not clear.2,3
Treatment for solitary plasmacytoma includes surgical resection and radiotherapy.4,5 In disseminated forms, such as multiple EMP, response is lower, prognosis is worse, and mean survival is reduced. In such cases, bortezomib-based systemic chemotherapy, bone marrow transplantation, or intensive regimens, such as DCEP or etoposide phosphate, prednisone, vincristine sulfate (Oncovin), cyclophosphamide, and doxorubicin hydrochloride (EPOCH), are employed. However, evidence mainly comes from individual cases and retrospective analyses, underlining the need for studies, to standardize treatment.6,7 Because of its rarity, treatment response and prognosis in patients with gastrointestinal involvement are difficult to evaluate. A retrospective analysis reported a serologic response rate of 67% and emphasized that organ involvement is a factor of poor prognosis.8 Another study found that patients with recurrent EMP had a progression-free survival of 9.1 months.9
Gastrointestinal bleeding is a challenging complication. Even though it usually manifests as chronic anemia, severe bleeding has been reported in gastric or duodenal lesions. In such settings, endoscopic treatment tends to have limited efficacy due to the tumor friability that predisposes to rebleeding, and so radiotherapy is prioritized. In refractory or surgically contraindicated cases, arterial embolization could be an alternative.10
The present case stands out because of the unusual location of the gastric plasmacytoma, its infrequency (less than 5% of EMPs), and its presentation in a patient with HIV, a condition that increases the speed of progression and the risk of dissemination.
In conclusion, there is no consensus on the management of multiple EMP with gastrointestinal involvement. A multidisciplinary approach is recommended that integrates surgery, hematology, oncology, pathology, and radiology. Options for personalizing treatment include surgical resection, radiotherapy, and systemic chemotherapy, with or without bone marrow transplantation.
Ethical considerationsApproval by the Bioethics Committee of the Pontificia Universidad Javeriana was not required, given that, according to the international ethics guidelines for research related to human health (CIOMS, version 2016), the present work is considered no-risk research. We declare that this article contains no personal information that could identify the patient.
Financial disclosureNo financial support was received directly or indirectly from any institution or person, in relation to this article.
The authors declare that there is no conflict of interest.