Total gastrectomy is utilized in different pathologies. Esophagojejunostomy leakage is a frequent complication. Our aim was to determine the association of the neutrophil-lymphocyte ratio (NLR) with esophagojejunostomy leakage that subsequently required invasive treatment.
Materials and methodsA retrospective study included patients that underwent esophagojejunostomy within the time frame of 2002-2017. Patients were grouped into those with or without anastomotic leakage that had conservative treatment (group A) and those with anastomotic leakage that had invasive treatment (group B). ROC curves and the Youden index were used for the optimum cutoff values of the NLR.
ResultsFifty-seven patients were included. Thirty-two (56.14%) were men, and mean patient age was 61.8 ± 13.4 years. Forty-five patients were assigned to group A and 12 to group B. Mean NLR was higher for group B on postoperative day 3 (group A 9.5 ± 7.5 vs. group B 13.9 ± 4.9) (p = 0.05). Mean total leukocytes was higher in group B on postoperative day 5 (group A 7.8 ± 3.4 × 103/mcl vs. group B 10.3 ± 4.4 × 103/mcl) (p = 0.03). NLR and total leukocyte accuracy on postoperative day 3 was calculated with ROC curves, at 0.78 and 0.63, respectively. For the NLR and leukocyte count, sensitivity was 91.7% and 58%, specificity was 64.4% and 60%, positive predictive value was 40% and 28%, and negative predictive value was 96% and 84%, respectively.
ConclusionsPostoperatively, the NLR identified the total gastrectomy with esophagojejunostomy patients that subsequently required an invasive procedure secondary to esophagojejunostomy leakage.
La gastrectomía total (GT) se utiliza en diversas patologías. La fuga anastomosis esófago yeyunal (FAEY) es una complicación frecuente. El objetivo es determinar la asociación del índice neutrófilo / linfocito (INL) con FAEY que requerirán tratamiento invasivo.
Material y MétodosEstudio retrospectivo, incluyó pacientes sometidos a GT en 2002-2017. Se agruparon los pacientes con o sin fuga de anastomosis que recibieron manejo conservador en un grupo (Grupo A), y pacientes con fuga que recibieron procedimiento invasivo en otro grupo (Grupo B). Se utilizaron curvas ROC y prueba de Youden para los valores de corte óptimos del INL.
ResultadosSe incluyeron 57 pacientes; 32(56.14%) eran hombres y la edad media fue 61.8 ± 13.4 años. Se asignaron 45 pacientes al Grupo A y 12 pacientes al Grupo B. La media de INL fue mayor para el Grupo B en el día 3 postoperatorio (PO)(9.5 ± 7.5 Grupo A vs 13.9 ± 4.9 Grupo B) (p = 0.05); la media de leucocitos totales fue mayor en el grupo B en el día 5 PO (7.8 ± 3.4 × 103/mcl Grupo A vs 10.3 ± 4.4 × 103/mcl Grupo B)(p = 0.03). La precisión del INL y los leucocitos totales en el 3PO fue calculada con curvas ROC, siendo 0.78 y 0.63 respectivamente. La sensibilidad fue de 91.7% y 58%, especificidad 64.4% y 60%, valor predictivo positivo 40% y 28% y valor predictivo negativo 96% y 84% para el INL y leucocitos respectivamente
ConclusionesEn el periodo postoperatorio, el INL predice cuales pacientes sometidos a GT con esófago yeyuno anastomosis requerirán algún procedimiento invasivo secundario a FAEY.
Total gastrectomy (TG) is a surgical procedure indicated for distinct clinical scenarios, in both benign and malignant pathologies. Its mortality rate has been reported at around 5.4% and deaths are associated with bleeding, sepsis, cardiorespiratory events, and thromboembolism.1
Currently, the most widely used technique for the reconstruction and reestablishment of the anatomy after TG is Roux-en-Y esophagojejunostomy.2 Esophagojejunostomy leakage (EJL) is the most dreaded complication during the early postoperative period, with a reported incidence of 12 to 31%.3
Small esophagojejunostomy leaks that do not cause a systemic inflammatory response or multiple organ failure can be managed conservatively with antibiotics, intestinal rest, and percutaneous drainage of intra-abdominal collections. On the other hand, large esophagojejunostomy leaks that require surgical reintervention or endoscopic procedures, such as stent placement to support anastomoses,4 are associated with an increase in the mortality rate.2,5 In patients with EJL, an estimated mortality rate of 19 to 64% is reported.6,7
The neutrophil-leukocyte ratio (NLR) has been proposed as an inflammatory response marker and a predictive instrument in different surgical procedures. Its usefulness as a predictor for the development of anastomotic leaks has been demonstrated in colorectal surgery,8 and has even been proposed as a determining survival factor in patients with colorectal cancer.9 In the context of upper digestive tract surgery, its utility has been validated as a predictor of postoperative complications, including abdominal sepsis, fistula formation, or pneumonia.10 However, its role in the specific prediction of esophagojejunostomy leaks in patients undergoing TG has not been specifically studied.
The aim of the present study was to determine the association of the NLR as an early marker of EJL that will require an invasive procedure, such as percutaneous puncture, endoscopy, or surgery, for its treatment.
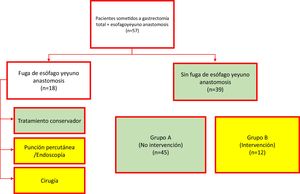
Materials and methodsA retrospective, descriptive, comparative study, with a correlational scope, was carried out on a cohort of cases. All patients that underwent TG within the time frame of 2002 to 2017 were included. Either mechanical or manual esophagojejunostomy was performed. When the procedure was mechanical, a 2-0 polypropylene tuck was made at the esophageal stump, a 21 mm circular stapler was utilized for the end-to-side anastomosis to the jejunum, and the jejunal stump was closed with a cutting curve stapler. When manual, the anastomosis was performed utilizing 2 layers, with continuous sutures of 4-0 polyglecaprone for the internal layer and simple stitches of 4-0 silk for the external layer. Data collection included demographic, clinical, pathologic, and laboratory variables, specifically the NLR and total leukocyte count on postoperative days 1, 3, and 5. EJL was defined as any sign of intraluminal fluid leakage from the esophagojejunostomy onto its exterior, documented through endoscopy, during re-operation, radiographic studies (contrast swallow with contrast extravasation or computed axial tomography with a peri-anastomotic collection), or the documentation of gastrointestinal fluid in the drains. For the statistical analysis, the cohort was divided into 2 groups. Group A was made up of the patients (with or without EJL) that only received conservative management and Group B was made up of the patients with EJL that received invasive treatment (puncture, endoscopy, or re-operation) (Fig. 1). The primary outcome to evaluate was the postoperative need for an invasive procedure.
Statistical analysisThe statistical analysis of the study variables was carried out according to their natural scaling and dispersion, utilizing the IBM® SPSS® Statistics version 21.0 program. The data graphics were created utilizing the Microsoft® Excel® version 14.7.7 program. The dimensional variables were contrasted with a 2-tailed Student’s t test and the categorical and ordinal variables were compared with the chi-square test. Any p value equal to or less than 0.05 or 5% (type I error) was considered statistically significant for a 2-tailed hypothesis test. ROC curves and the Youden test were employed to calculate the optimal cutoff values. Once the cutoff values were established, sensitivity, specificity, positive predictive value, and negative predictive value were calculated for the NLR and total leukocyte count.
Ethical considerationsThe present work met all the codes of ethics in human research, according to the Asociación Mexicana de Cirugía General, the Declaration of Helsinki, and the Federal Law for the Protection of Personal Data. It was also submitted to the institutional human research bioethics committee.
ResultsA total of 57 patients were included in the study. The indication for TG was gastric adenocarcinoma or gastroesophageal junction adenocarcinoma, in all cases. Mechanical esophagojejunostomy was performed on 45 (78.9%) patients and manual esophagojejunostomy on 12 (21.1%). EJL was diagnosed in 18 patients, 6 of whom (33.3%) were treated conservatively, whereas the remaining 12 patients (66.7%) underwent invasive treatment (percutaneous puncture or surgery). As a result, 45 patients were assigned to the non-intervention group (Group A) and 12 patients were assigned to the intervention group (Group B). The mean ± SD of patient age was 61.8 ± 13.4 years and was similar in the 2 groups: 60.8 ± 14.6 years for Group A and 65.9 ± 12.8 years for Group B (t test, p = 0.246). Regarding sex distribution, 32 patients were men (56.14%). The most frequent comorbidities were high blood pressure and type 2 diabetes mellitus. Table 1 summarizes the demographic and clinical characteristics of the study patients.
Demographic and clinical characteristics of the patients
| Total (n = 57) | No intervention (n = 45) | Intervention (n = 12) | p | |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.8 ± 13.4 | 60.8 ± 14.6 | 65.9 | 0.246a |
| Sex, n (%) | 0.564 | |||
| Men | 32 (56.14%) | 25 (55.55%) | 7 (58.33%) | |
| Women | 25 (43.86%) | 20 (44.45%) | 5 (41.67%) | |
| Comorbidities, n (%) | ||||
| HBP | 20 (35%) | 15 (33.33%) | 5 (41.66%) | 0.41b |
| DM2 | 17 (29.8%) | 11 (24.44%) | 6 (50%) | 0.089b |
| COPD | 1 (1.75%) | 1 (2.22%) | 0 | 0.789b |
| Obesity | 2 (3.5%) | 1 (2.22%) | 1 (8.33%) | 0.38b |
HBP: high blood pressure; DM2: diabetes mellitus type 2, COPD: chronic obstructive pulmonary disease.
Regarding tumor characteristics, the majority of patients (45.61%), overall, were in advanced clinical stages, i.e., stages III or IV. That similarity of clinical stage distribution was maintained when the cohort was divided into Group A and Group B. Table 2 summarizes the histologic, preoperative, intraoperative, and postoperative patient characteristics. Interestingly, all the characteristics were similar in the 2 groups, except for hospital stay. The mean ± SD for hospital stay was 22.2 ± 19.06 days and was significantly higher in Group B than in Group A (40.4 ± 30.7 days in Group B vs. 17.2 ± 10.2 days in Group A; t test, p < 0.001).
Histologic, preoperative, intraoperative, and postoperative characteristics of the patients
| Total (n = 57) | No intervention (n = 45) | Intervention (n = 12) | p | |
|---|---|---|---|---|
| Clinical stage, n (%) | 0.837b | |||
| I | 3 (5.26%) | 2 (4.44%) | 1 (8.33%) | |
| II | 13 (22.8%) | 11 (24.44%) | 2 (16.66%) | |
| III | 14 (24.56%) | 10 (22.22%) | 4 (33.33%) | |
| IV | 12 (21.05%) | 7 (15.55%) | 5 (41.66%) | |
| Chemotherapy, n (%) | 0.699b | |||
| No chemotherapy | 19 (33.33%) | 14 (31.11%) | 5 (41.66%) | |
| Neoadjuvant | 11 (19.29%) | 8 (17.77%) | 3 (25%) | |
| Adjuvant | 10 (17.54%) | 9 (20%) | 1 (8.33%) | |
| Perioperative | 17 (29.82%) | 14 (31.11%) | 3 (25%) | |
| Histology (n = 47), n (%) | 0.717b | |||
| Intestinal | 13 (27.65%) | 11 (30.55%) | 2 (18.18%) | |
| Diffuse | 27 (57.44%) | 20 (55.55%) | 7 (63.63%) | |
| Mixed | 7 (14.89%) | 5 (13.88%) | 2 (18.18%) | |
| Surgery duration (min), mean ± SD | 275 ± 58.3 | 276.8 ± 61.9 | 268.3 ± 44.2 | 0.59a |
| Blood loss (ml), mean ± SD | 503.5 ± 246.8 | 514 ± 255 | 465 ± 217 | 0.547a |
| Anastomosis, n (%) | 0.46b | |||
| Manual | 8 (14.03%) | 7 (15.55%) | 1 (8.33%) | |
| Mechanical | 49 (85.96%) | 38 (84.44%) | 11 (91.66%) | |
| Hospital stay (days), mean ± SD | 22.2 ± 19.06 | 17.2 ± 10.2 | 40.4 ± 30.7 | 0.001a |
SD: standard deviation.
The leukocyte level and NLR data were collected for the two groups on postoperative days 1, 3, and 5 (Table 3).
Neutrophil-leukocyte ratio and total leukocytes in both groups
| Group A mean ± SD | Group B mean ± SD | p | ||||
|---|---|---|---|---|---|---|
| NLR | Leukocytes | NLR | Leukocytes | NLR | Leukocytes | |
| Postoperative day 1 | 11.1 ± 8.8 | 11.1 ± 4.4 | 16.9 ± 10.8 | 11.1 ± 2.8 | 0.06a | 0.96a |
| Postoperative day 3 | 9.5 ± 7.5 | 8.6 ± 3.9 | 13.9 ± 4.9 | 10.8 ± 4.8 | 0.05a | 0.11a |
| Postoperative day 5 | 7.6 ± 7.9 | 7.8 ± 3.4 | 14.3 ± 10.9 | 10.3 ± 4.4 | 0.01a | 0.03a |
SD: standard deviation.
On postoperative day 1, there was no statistically significant difference in the mean NLR and total leukocyte comparison between the 2 groups. The mean NLR was 11.1 in Group A vs. 16.9 in Group B (t test, p = 0.06) and the mean total leukocyte count was 11.1 × 103/μl for both groups (t test, p = 0.96).
On postoperative day 3, there was a significant difference in the mean comparison of the NLR values but not in the total leukocyte comparison. The mean NLR was 9.5 in Group A vs. 13.9 in Group B (t test, p < 0.05), whereas the mean total leukocyte level was 8.6 × 103/μl in Group A vs. 10.8 × 103/μl in Group B (t test, p = 0.11).
On postoperative day 5, the mean comparison produced statistically significant differences for both the NLR and total leukocytes. The mean NLR was 7.6 in Group A vs. 14.3 in Group B (t test, p = 0.01) and the mean total leukocyte level was 7.8 × 103/μl in Group A vs. 10.3 × 103/μl in Group B (t test, p = 0.03).
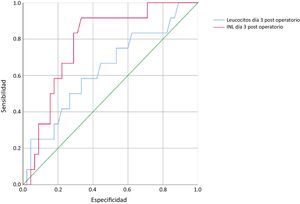
Upon finding a statistically significant difference in the mean NLR at postoperative day 3, ROC curves were plotted for the NLR and total leukocyte values on that day, revealing an area of 0.78 for the NLR and 0.63 for the total leukocytes (Fig. 2).
The Youden test was employed to establish the optimal cutoff points for each of the 2 tests on postoperative day 3. The optimal cutoff point was 10 for the NLR and 9 × 103/μl for the total leukocyte levels. With the established cutoff values, sensitivity was 91.7% for the NLR and 58% for total leukocytes, specificity was 64.4% for the NLR and 60% for total leukocytes, the positive predictive value was 40% for the NLR and 28% for total leukocytes, and the negative predictive value was 96% for the NLR and 84% for total leukocytes.
DiscussionEarly identification of the complications associated with TG, specifically EJL, contributes to opportune management and reduced morbidity and mortality. To achieve that early identification, in the development of the present work, we proposed utilizing the NLR as an incipient marker for EJL. We found that, unlike total leukocytes, the NLR was significantly higher in the patients that ended up having invasive procedures. The use of that inflammation marker on postoperative day 3 had an area under the curve of 0.78, making it a so-called adequate test, whereas the total leukocyte level had an area under the curve of 0.63, resulting in a test with poor performance for diagnosing EJL.11 The optimal cutoff values were determined using the Youden index,12 and were 10 for the NLF and 9 × 103/μl for the total leukocyte count, resulting in higher sensitivity and negative predictive values for the NLR than for total leukocytes.
Numerous studies have attempted to associate the use of different biomarkers with the development of anastomotic leakage after TG with esophagojejunostomy. In a systematic review that included 24 articles, Maarten de Mooij et al.13 found that the majority of the studies concentrated on the use of C-reactive protein (CRP) and leukocytes, and other biomarkers utilized were prealbumin, procalcitonin, albumin, and interleukin (IL)-6, IL-8, and IL-10. The areas under the curve in all those studies varied from 0.48 to 0.99, depending on the biomarker used and the postoperative day they were determined. A study by Ji et al.14 utilized CRP, and an area under the curve of 0.99 was reached with very high cutoff points.14
Similar to our study, Çetin et al.15 conducted a correlational study for EJL diagnosis and CRP was the marker that showed the earliest elevation, whereas the NLR increased significantly, but later, i.e., on postoperative day 5. Those authors did not report areas under the curve or sensitivities for the markers they analyzed.
The heterogenicity of the EJL diagnoses among all the studies in the literature is interesting and can be clearly appreciated in the abovementioned systematic review, in which there are 24 different definitions of EJL.13 Therefore, in an effort to homogenize the data from the different reports in the surgical literature, we find it relevant to extrapolate the definitions established by the Esophagectomy Complications Consensus Group (ECCG), for their use in the reports on gastric surgery. That consensus defines anastomotic leak as a full thickness gastrointestinal tract defect that includes the anastomosis or staple line, regardless of the presentation or method of identification. Likewise, leaks are divided into type 1, in which the defect is local, and the treatment is medical/conservative; type 2, in which the defect requires interventional therapy but no re-intervention (percutaneous puncture, endoscopy, stent placement); and type 3, in which the defect requires re-operation to be resolved.16 Utilizing those definitions, in our study, the Group A patients presented with type 1 leak, whereas the Group B patients presented with type 2 or type 3 leaks. In other words, according to the findings of the present study, the NLR is an early marker for the development of type 2 or type 3 EJL.
The findings of the present analysis are very useful in clinical practice. Through the opportune and postoperative identification of the patient that can develop a complication, such as EJL, after having undergone TG, we can perform interventions focused on improving outcome. Those interventions can be as simple as prolonging postoperative fasting and keeping the postoperative drains in place, or they can include starting antibiotic therapy and/or total parenteral nutrition, all of which aims to let the anastomosis rest and promote its adequate cicatrization.
The weaknesses of the present study are its retrospective design and the small sample size. The retrospective design did not allow us to evaluate other early inflammation markers that would be interesting to assess, such as CRP and procalcitonin. In that sense, a prospective work that obtained the values of those biomarkers and developed scales that included a combination of different biomarkers would be of interest.
Opportune diagnosis of EJL should definitely be accompanied by a careful analysis of the risk factors leading the patient to develop said complication, such as the technical difficulty for creating the anastomosis or patients with respiratory failure.17 Likewise, the correct use of the different imaging techniques, such as esophagograms or computed axial tomography, can aid in the diagnosis of EJL.18
ConclusionsThe NLR was shown to be an inflammation marker that increased early on, in postoperative TG patients with esophagojejunostomy that developed type 2 or type 3 leaks. Unlike other newer markers, such as procalcitonin and different interleukins, the NLR is a simple, rapid, and inexpensive test. Given its good sensitivity and excellent negative predictive value, it could contribute to the opportune treatment of patients with EJL.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Clemente-Gutiérrez U, Sarre-Lazcano C, Casanueva-Pérez E, Sánchez-Morales G, Mier y Terán-Ellis S, Contreras-Jiménez E, et al. Utilidad de marcadores de inflamación para detectar fugas de anastomosis esofagoyeyunal. Revista de Gastroenterología de México. 2021;86:229–235.