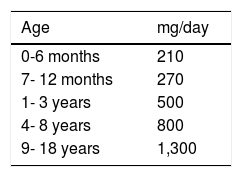Celiac disease, celiac sprue, or gluten-sensitive enteropathy, is a generalized autoimmune disease characterized by chronic inflammation and atrophy of the small bowel mucosa. It is caused by dietary exposure to gluten and affects genetically predisposed individuals. In Mexico, at least 800,000 are estimated to possibly have the disease, prompting the Asociación Mexicana de Gastroenterología to summon a multidisciplinary group of experts to develop the “Clinical guidelines on the diagnosis and treatment of celiac disease in Mexico” and establish recommendations for the medical community, its patients, and the general population. The participating medical professionals were divided into three working groups and were given the selected bibliographic material by the coordinators (ART, LUD, JMRT), who proposed the statements that were discussed and voted upon in three sessions: two voting rounds were carried out electronically and one at a face-to-face meeting. Thirty-nine statements were accepted, and once approved, were developed and revised by the coordinators, and their final version was approved by all the participants. It was emphasized in the document that epidemiology and risk factors associated with celiac disease (first-degree relatives, autoimmune diseases, high-risk populations) in Mexico are similar to those described in other parts of the world. Standards for diagnosing the disease and its appropriate treatment in the Mexican patient were established. The guidelines also highlighted the fact that a strict gluten-free diet is essential only in persons with confirmed celiac disease, and that the role of gluten is still a subject of debate in relation to nonceliac, gluten-sensitive patients.
La enfermedad celiaca (EC), esprúe celíaco o enteropatía sensible al gluten, es una enfermedad autoinmune generalizada que se caracteriza por inflamación crónica y atrofia de la mucosa del intestino delgado, causada por la exposición al gluten de la dieta que afecta a individuos genéticamente predispuestos. En México se estima que al menos 800,000 personas podrían padecerla, por lo que la Asociación Mexicana de Gastroenterología convocó a un grupo multidisciplinario de expertos para que realizaran la Guía clínica para diagnóstico y tratamiento de enfermedad celíaca en México, y se establecieran recomendaciones para la comunidad médica, sus enfermos y la población general. Los profesionistas participantes, divididos en 3 mesas de trabajo, recibieron material bibliográfico seleccionado por los coordinadores (ART, LUD, JMRT), quienes propusieron los enunciados que fueron discutidos y votados en 3 sesiones: 2 a través de medios electrónicos y una presencial. Al final se aceptaron 39 enunciados que, una vez aprobados, fueron desarrollados y revisados por los coordinadores hasta su versión final, que fue aprobada por todos los participantes. Dentro de estas se destaca que la epidemiología y factores de riesgo asociados (familiares de primer grado, enfermedades autoinmunes, poblaciones de alto riesgo) a EC en México son similares a los descritos en otras partes del mundo. Se establecen pautas para el diagnóstico y el tratamiento apropiado de los pacientes mexicanos que la padecen. Se insiste en que una dieta estricta libre de gluten es indispensable solo en las personas con EC confirmada, y que su papel en pacientes con sensibilidad al gluten sin EC es aún un tema de controversia.
Celiac disease (CD), celiac sprue, or gluten-sensitive enteropathy, is a generalized autoimmune disease characterized by chronic inflammation and atrophy of the small bowel mucosa caused by dietary gluten exposure that affects genetically predisposed individuals. 1–2
Even though CD was considered rare in Mexico, relevant information has come out over the last ten years with respect to its epidemiology, and it is estimated that between 800,000 and 1,000,000 Mexicans could present with the disease.3–4 New evidence has been produced at the international level on its diagnosis and treatment, motivating the Asociación Mexicana de Gastroenterología to summon a group of medical professionals interested in the theme to develop the “Clinical guidelines for the diagnosis and treatment of celiac disease in Mexico” and propose useful recommendations for the medical community and nutritionists on this subject of undeniable topicality.
Our aim was to provide an updated document on the epidemiology, diagnosis, and treatment of CD in Mexico. The recommendations are based on an extensive review of the literature and the consensus opinion of experts.
MethodsTo develop the present guidelines, three designated coordinators (LFUD, JMRT, and ART) organized a multidisciplinary group of gastroenterologists (adult and pediatric), endoscopists, pathologists, and nutritionists divided into 3 working groups to discuss: 1) definition and epidemiology, 2) diagnosis, and 3) vigilance and treatment. The coordinators carried out a detailed search in the following databases: CENTRAL (The Cochrane Central Register of Controlled Trials), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, Bio Med Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP) within the time frame of January 1, 2010 and February 28, 2017. The criteria included the terms: “enfermedad celiaca/celiac disease” combined with: “epidemiology”, “incidence”, “prevalence”, “Mexico”, “gluten”, “sensitivity”, “diagnosis”, “differential diagnosis”, “treatment”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis”, and their Spanish equivalents. All the bibliographic material was made available to the collaborators for their review at any time throughout the process.
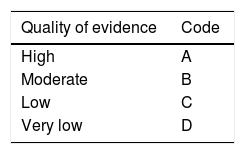
Each working group identified the areas of clinical relevance and reviewed the bibliographic material provided to identify the evidence. They formulated pertinent statements that were electronically reviewed by each of the members of the corresponding working group on two occasions. Finally, the statements were presented, discussed, and voted on at a face-to-face meeting that took place on September 7, 2017, in Tijuana, Baja California. All the collaborators voted on the statements based on three possible answers: utilizing the a) “in complete agreement”, b) “in partial agreement”, and c) “in disagreement”. The statements that had a total of “complete agreement”> 75% were kept and those that had a total of “disagreement”> 75% were eliminated. Once it was established which statements would be included in the guidelines, the coordinators formulated the first version of the document, utilizing the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to give a strength of recommendation and quality of evidence grade to each statement.5 According to that system, the grading of quality of evidence is based not only on study design or methodology, but also on a clearly posed question in relation to an outcome variable that must also be clearly stated. Based on those criteria, the level of evidence can be graded as high, moderate, low, or very low. In addition, the strength of recommendation is graded as strong or weak, in favor of or against, the intervention or statement.6 The GRADE system utilizes a code in which an upper-case letter describes the quality of evidence, followed by a number that indicates the strength of recommendation (Table 1).
GRADE system coding. Classification of the quality of evidence and strength of recommendations.
| Quality of evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of recommendation | Code |
|---|---|
| Strong in favor of the intervention | 1 |
| Weak in favor of the intervention | 2 |
| Weak against the intervention | 2 |
| Strong against the intervention | 1 |
The final version of the document was approved by all the participants.
ResultsDefinition, classification, and epidemiologic notes1. Celiac disease (CD) is an autoimmune disease triggered by the ingestion of gluten and related proteins that affects persons with genetic susceptibility.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
CD, also known as celiac sprue, or gluten-sensitive enteropathy, is defined by intolerance to wheat gliadins and prolamins contained in other cereals that harm the mucosa of the small bowel to varying degrees in genetically susceptible individuals.7 Genetic susceptibility is conferred by the presence of the HLA-DQ2 histocompatibility allele with the typical DQA1*0501/DQB1*0201 heterodimer present in 95% of the individuals and the HLA-DQ8 allele with the HLA-DQB1*0302 heterodimer in the remaining 5%.8 Even though the presence of those alleles is indispensable for the diagnosis of the disease, the interaction of other environmental factors is required for its development.7 The autoimmune phenomenon is corroborated by demonstrating a cell response mediated by CD4+lymphocytes 9 and the presence of anti-tissue transglutaminase autoantibodies (anti-tTG and tTG2), which are proteins that belong to a family of calcium-dependent cytoplasmic enzymes.10 Gluten is known to produce tissue damage and increase permeability, resulting in the release of cytosolic tTG into the intracellular space, to later be presented to the CD4+T-lymphocytes by the specialized cells of the major histocompatibility complex.11
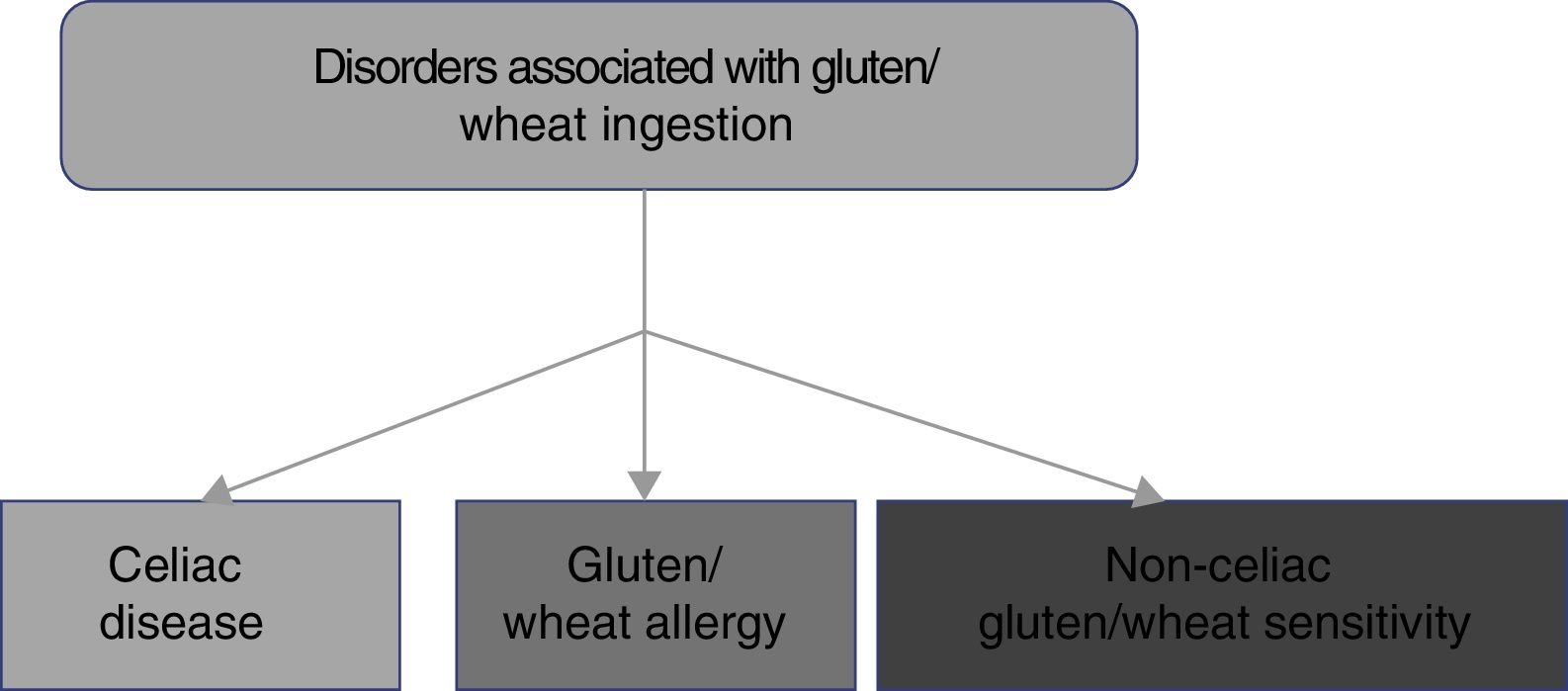
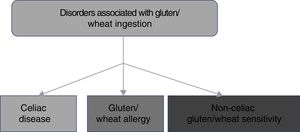
2. CD belongs to the disorders related to gluten ingestion, which include gluten/wheat allergy and non-celiac gluten/wheat sensitivity.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 89%, in partial agreement 11%).
The consumption of foods that contain gluten has been and continues to be of the utmost importance for human alimentation. However, there has been a recent increase in the prevalence of disorders related to the ingestion of gluten/wheat estimated to affect around 5% of the world population.1 Multiple mechanisms have been linked to the physiopathogenesis of gluten-related disorders, some of which are well-defined, whereas others are still subjects of debate (fig. 1). The current classification of gluten-related disorders is evolutionary. The activation of T-lymphocytes is recognized in CD to be triggered by exposure to gluten, present in foods that contain wheat, barley, and rye.1-2 On the other hand, non-celiac gluten sensitivity (NCGS) allergies can be related to proteins other than gluten, therefore experts have recommended that those disorders be classified as gluten/wheat-related.12 Allergy to wheat components presents as an IgE-associated immune reaction that triggers an inflammatory response against the alpha-amylase/trypsin inhibitors, nonspecific lipid transfer protein (nsLTP), gliadins, and high-molecular-weight glutenin. That allergy is epidemiologically more frequent in the pediatric population.2 NCGS is characterized by the presence of intestinal and extraintestinal symptoms after the ingestion of foods containing gluten/wheat, and its pathophysiology is not completely understood.13 A group of experts defined criteria for diagnosing NCGS (Salerno criteria) that at present are the best protocol for its diagnosis. The definitions are expected to be adjusted in the future, as the pathophysiologic mechanisms of those disorders become recognized.
3. The estimated prevalence of CD in Mexico is 0.5-0.7%.
Quality of evidence and strength of recommendation: B2 weak in favor of the statement (in complete agreement 100%).
CD has a worldwide distribution, with an estimated prevalence between 1:67 and 1:250. Prevalence may be underestimated in certain areas, especially in Latin American populations.14,15 Seroprevalence of anti-tTG and anti-endomysial (EMA) antibodies has been calculated at 0.59% (0.27-1.29%) in a Mexican Mestizo population, mainly associated with the HLA genotype with DQ8 predominance.3,4,16-18 Those observations indicate that the prevalence and genetic susceptibility in the Mexican Mestizo population are similar to those of other populations.
4. There are groups of persons at higher risk for CD and therefore subject to screening.
Quality of evidence and strength of recommendation:B1 strong in favor of the statement(in complete agreement 94%, in partial agreement 6%).
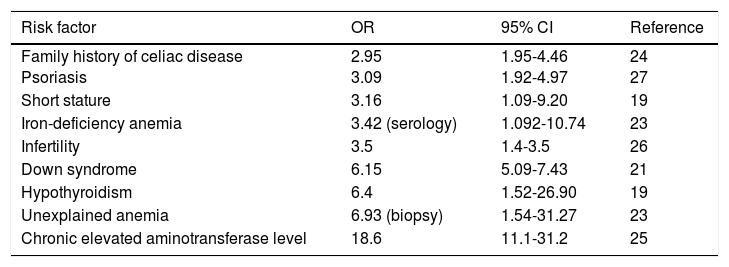
Studies have shown the association of CD with certain conditions and when they are present, the suggestion is to carry out screening tests to rule out CD. Those conditions include: first and second-degree relatives with celiac disease, autoimmune diseases (type I diabetes mellitus, autoimmune thyroiditis, Addison's disease),19-20 Down syndrome,21 Turner syndrome,22-23 unexplained iron-deficiency anemia,24 unexplained elevation of aminotransferases,25 osteopenia and unexplained osteoporosis,2 neuropathy, migraine, ataxia, dermatitis herpetiformis, psoriasis,26 short stature, dental enamel hypoplasia, primary infertility,27 Williams-Beuren syndrome, and selective IgA deficiency (Table 2).2,12 Nevertheless, a recent case-control study showed that detection through that type of strategy (identifying high-risk groups, except for hypothyroidism) was not effective in the majority of patients with undiagnosed CD.28 Thus, better strategies for identifying new cases are required.
Populations at risk for celiac disease and considered for screening.
| Risk factor | OR | 95% CI | Reference |
|---|---|---|---|
| Family history of celiac disease Psoriasis | 2.95 3.09 | 1.95-4.46 1.92-4.97 | 24 27 |
| Short stature | 3.16 | 1.09-9.20 | 19 |
| Iron-deficiency anemia | 3.42 (serology) | 1.092-10.74 | 23 |
| Infertility | 3.5 | 1.4-3.5 | 26 |
| Down syndrome | 6.15 | 5.09-7.43 | 21 |
| Hypothyroidism | 6.4 | 1.52-26.90 | 19 |
| Unexplained anemia | 6.93 (biopsy) | 1.54-31.27 | 23 |
| Chronic elevated aminotransferase level | 18.6 | 11.1-31.2 | 25 |
5. The frequency of CD in some higher-risk groups studied in Mexico is similar to that reported in other countries.
Quality of evidence and strength of recommendation: B2 weak in favor of the statement (in complete agreement 100%).
The prevalence of CD in Mexico is similar to that reported in other countries, including that found in high-risk populations, such as patients with type I diabetes mellitus, in whom prevalence was 5.9% (serology and histology) in Mexican adults,29 and between 9% and 12% in the pediatric population.30 Other at-risk populations in whom prevalence was determined were: patients with monosomy 7 or Williams-Beuren syndrome (23%),31 autoimmune liver diseases (6.6%),32 and persons with unexplained infertility (3-5%).33
Prevalence of CD in Mexican patients with diarrhea-prominent and mixed type irritable bowel syndrome (IBS), according to the Rome III criteria, has been reported to vary between 2.25% and 3.5%, compared with 0.5% in the general population (OR: 5.21; 95% CI: 1.13-2.93; p=0.04).34 The same group of researchers stated that up to 6% of Mexican patients with uninvestigated dyspepsia can present with CD.35
6. Depending on its presentation, CD can be classified as: 1) Symptomatic; 2) Asymptomatic; and 3) Potential.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
The signs and symptoms of celiac disease were described more than 100 years ago. Over such an extended period of time, research on and knowledge of the disease has greatly increased.2,12. The evolution in knowledge has been accompanied by the use of many terms and classifications, which has led to confusion and a lack of consensus. In that context, and according to definitions that have been published by a multidisciplinary study group, the proposed clinical classification of CD is 1) Symptomatic; 2) Asymptomatic; and 3) Potential.
7. Symptomatic CD can present with gastrointestinal manifestations (classic CD) or extraintestinal symptoms.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
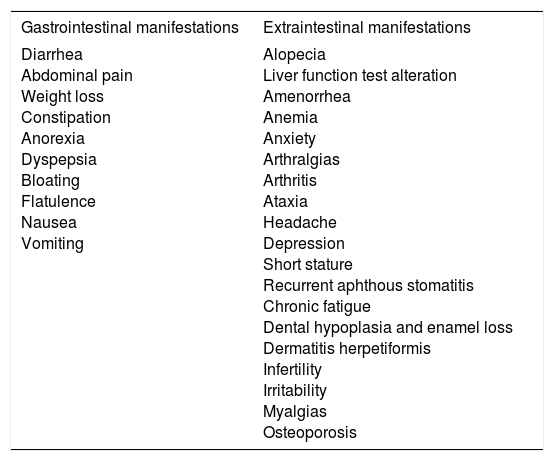
Symptomatic CD is characterized by the presence of gastrointestinal or extraintestinal symptoms (Table 3) that can clearly be associated with gluten ingestion.36-37 That category can include patients presenting with the classic disease symptoms of diarrhea, steatorrhea, weight loss, or delayed growth. The term “typical celiac disease” should be avoided because it alludes to frequency, and there is increasing evidence that what are considered classic symptoms are not the most common. Therefore, the term “symptomatic celiac disease” is preferred. Diagnosis requires a high rate of suspicion, given that under 50% of adults present with gastrointestinal symptoms.38 The development of highly sensitive and specific noninvasive tests has improved recognition of the disease and its identification in at-risk subjects.39 Gastrointestinal symptoms are the most common form of presentation in very young children, in whom CD generally presents as vomiting, abdominal pain, diarrhea, and refractory constipation, whereas extraintestinal symptoms present in older children and adolescents.40
Clinical manifestations of symptomatic celiac disease.
| Gastrointestinal manifestations | Extraintestinal manifestations |
|---|---|
| Diarrhea Abdominal pain Weight loss Constipation Anorexia Dyspepsia Bloating Flatulence Nausea Vomiting | Alopecia Liver function test alteration Amenorrhea Anemia Anxiety Arthralgias Arthritis Ataxia Headache Depression Short stature Recurrent aphthous stomatitis Chronic fatigue Dental hypoplasia and enamel loss Dermatitis herpetiformis Infertility Irritability Myalgias Osteoporosis |
Non-classic CD is part of symptomatic CD and is characterized by the lack of malabsorption data (e.g., persons with abdominal pain or constipation).
8. The classic manifestations of CD are bloating and abdominal pain, borborygmi, diarrhea with deficient nutrient absorption, weight loss, and general malaise.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 94%, in partial agreement 6%).
Historically, classic CD has been defined by the presence of diarrhea, malnutrition, or malabsorption syndrome (weight loss, steatorrhea, and edema secondary to hypoalbuminemia).2 We now know that the classic CD symptoms are less frequent.35 Even though not all of the symptoms are considered “classic” today, the most common ones include bloating and abdominal pain, borborygmi, diarrhea with deficient nutrient absorption (such as iron deficiency), weight loss, general malaise, chronic fatigue, and osteoporosis.38-39,41 The prevalence of clinical manifestations varies according to different factors such as the population studied (e.g., the youngest children present mainly with gastrointestinal symptoms and delayed growth, whereas adolescents have a greater number of extraintestinal manifestations), the operational definitions employed, and the era in which they were studied.42 At present, CD is often monosymptomatic, with less severe symptoms and intestinal pathology.
A study conducted at 2 Mexican referral centers in which 80 Mexican patients with CD were evaluated, highlighted the fact that the most common manifestation was diarrhea (86%), followed by bloating (77%) and abdominal pain (71%). The mean duration of clinical manifestations before diagnosis was 10 years, and strikingly, IBS had been previously diagnosed in 64% of the patients.43
9. Asymptomatic CD is characterized by: 1) positive celiac autoimmunity serology; 2) alterations in the biopsy of the duodenal mucosa; and 3) absence of apparent signs and symptoms.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
CD is called asymptomatic when there are no symptoms at the time of initial diagnosis, even when the clinical history included directed questions. Patients with asymptomatic CD are those identified through detection programs in countries with a high prevalence of the disease or conditions associated with a high risk for CD.
In some cases, symptoms are discreet, and the patient has had them throughout his or her lifetime, and therefore does not think of them as pathologic until they disappear upon suspending gluten consumption. In children, the asymptomatic disease variant can only be characterized by short stature for age, whereas adolescent girls can present with mild anemia or osteopenia, especially after menarche.
Positive serology refers to the detection of anti-deamidated gliadin peptide (DGP) antibodies, anti-endogenous protein antibodies, such as tTG, and/or endomysial antibody (EMA). Histologic alterations can include shortening of the intestinal villi and increased intraepithelial lymphocytes.2,40
10. Potential CD presents in persons with normal intestinal biopsy that are at high risk for developing CD due to the presence of the anti-DGP, anti-tTG, and/or anti-EMA antibodies.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
Individuals with normal small bowel mucosa that are at increased risk for developing CD due to presenting with positive serology are patients with potential CD. The term “latent celiac disease” should be avoided. There are at least 5 definitions of that term, leading to much confusion. 2,12
Patients with potential CD should be tested for the presence of the HLA-DQ2/DQ8 haplotypes to rule out other causes that could explain the finding of CD-associated antibodies.24. In addition, it is important to repeat antibody testing at least 3 months after the first positive result, to reaffirm the presence of potential CD. At any rate, antibody testing should be performed twice a year in adult patients with potential CD, and perhaps more frequently in children with that variant. Treatment with a gluten-free diet can be considered, especially in patients with positive anti-EMA antibodies because it can prevent the development of symptoms and damage to the duodenal mucosa.
11. CD that does not respond to treatment is characterized by symptom persistence, signs of nutrient deficiencies, or alterations in laboratory test results, despite following a strict gluten-free diet for 6 months.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 94%, partially in agreement 6%).
Symptom persistence or recurrence despite following a gluten-free diet is observed in around 30% of patients with celiac disease.44 The first step in the evaluation of those patients is making sure that the diagnosis of CD is correct. That involves a detailed review of the type of antibody that gave the positive result and of the duodenal biopsy slides.45 The next step is the thorough evaluation of the possible food sources of gluten contamination carried out by an expert nutritionist.46 The systematic evaluation of other causes (statement 36) not related to dietary adherence is indicated if gluten contamination was ruled out in the initial evaluation.47 Once potential causes have been ruled out, the presence of severe malabsorption, progressive malnutrition, and persistent intestinal villous atrophy suggest refractory CD.
12. Refractory CD is a rare variant defined by symptom persistence or recurrence and signs and symptoms suggestive of malabsorption, with intestinal villous atrophy, despite following a strict gluten-free diet for 12 months, in the absence of any other possible cause of the symptoms.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
Refractory CD is rare and affects at least 2% of celiacs.48 It is more frequent in men diagnosed in adulthood. It is exceptional in individuals under 30 years of age. Hypoalbuminemia is a poor outcome factor.49 Autoantibodies (tTG and EMA) are often negative.50 Diagnosis requires the systematic exclusion of other more common causes of nonresponse to the gluten-free diet.
13. There are 2 types of refractory CD according to immunophenotype: type I, which is immunologically mediated; and type II, characterized by clones of monoclonal intraepithelial lymphocytes. Type I responds well to medical treatment and type II has poor prognosis due to its association with severe malnutrition and intestinal lymphoma.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
Refractory CD is classified by the presence of abnormal T-cell clones in the intestinal mucosa. Clones should be searched for through special molecular studies, immunohistochemistry or flow cytometry, and in intestinal biopsies.51 Abnormal T-cell clones express the CD3 marker in cytoplasm but lose CD8 marker expression on the surface. They also show T-cell gamma receptor clonal re-arrangement. The presence of abnormal T-cells defines type II refractory CD, which responds inadequately to the available treatments, increases the risk for transformation to lymphoma, and has an elevated mortality rate.52 Patients with type I refractory CD meet the clinical criteria for refractory CD without the abnormal T-cell clone. Type I refractory CD has a better prognosis because it responds favorably to medical treatment with steroids, such as budesonide and/or immunosuppressants (e.g., azathioprine).53 Total parenteral nutrition should be considered in patients with severe malnutrition. The treatment of type II refractory CD should be individualized, and it is recommendable to refer those patients to a specialized center.
Diagnosis14. The basis for diagnosing CD is its contemplation in the appropriate clinical context. Accurate diagnosis includes the combination of clinical history, serology, and biopsy of the duodenal mucosa.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
A basic principle in medical practice is: “If you don’t think of it, you won’t look for it; if you don’t look for it, you won’t find it”. What is not suspected cannot be diagnosed. CD is a clear example of that concept. Usually included in the differential diagnosis of patients with malabsorption syndrome, clinical manifestations of CD that affect other organs and systems go unnoticed and presently dominate the clinical scenario in the majority of persons that present with the disease.2,40 Thus, diagnostic suspicion must increase, and the disease should be directly looked for in other entities that do not usually present with disturbances attributable to the gastrointestinal tract.
At any rate, accurate diagnosis does not depend on one isolated datum and it is necessary to combine clinical aspects with laboratory results, especially autoantibody testing and histologic alterations that are consistent with CD, albeit not exclusive of the disease.
15. In persons 2 years old or older, screening through IgA anti-tissue transglutaminase antibody (anti-tTG IgA) testing is suggested. In children with negative anti-tTG IgA but with symptoms suggestive of CD (especially if they are under 2 years of age), anti-DGP IgG and/or IgA (anti-DGP IgA/IgG) testing is proposed.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 95%, in partial agreement 5%).
The development of serologic tests for diagnosing CD began around 1980 with the introduction of native anti-gliadin antibodies, which are no longer in use due to their low sensitivity and specificity. The anti-reticulin antibodies appeared soon thereafter, and their use has also been discontinued, thanks to better results with other assays developed almost simultaneously. Currently, anti-EMA testing provides the most specificity, but it requires specialized personnel and technology that is not always available in the Mexican medical environment. 2,40
The discovery that tissue transglutaminase was the antigen that anti-EMA antibodies acted against led to the development of better techniques that have made it possible for practically any clinical laboratory to quantify anti-tTG IgA and IgG antibodies. In contrast to anti-EMA testing, the quantification of those antibodies is less expensive, objective, and not subject to personal interpretation.
Hopper et al.54 compared the performance of anti-tTG antibody quantification with anti-tTG and anti-EMA antibody sequential quantification. The negative prediction values were similar between both strategies (99.6% vs. 99.7%), but sensitivity was slightly greater with the single measurement of anti-tTG (90.9% vs. 85.7%). Based on its relative ease of performance, accessibility, and cost, most clinical guidelines recommend beginning the study of a person suspected of having CD with anti-tTG IgA antibody testing.2,40
A laboratory method that employs deamidated gliadin peptide (DGPs) as the antigen has recently been developed. Because DGPs are more immunogenic, initial results were promising. However, there is no advantage of anti-DGP IgA and IgG antibodies over anti-tTG IgA antibodies, except under 2 specific clinical conditions: in persons with selective IgA deficiency and in children under 2 years of age.2,10,12
16. If anti-tTG IgA antibodies are negative but CD suspicion is high, selective IgA deficiency should be ruled out, and if confirmed, anti-tTG IgG or anti-DGP IgG testing should be ordered.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 95%, in partial agreement 5%).
Even though the diagnostic yield of blood tests is quite high, there are 2 conditions in which false negatives can occur: 1) selective IgA deficiency and 2) when tests are performed in a patient following a gluten-free diet or in patients taking immunosuppressants.
Selective IgA deficiency is noticeably higher in persons with CD.2,10,40 A common practice in several parts of the world is to begin the study of the patient suspected of having CD by quantifying serum IgA. If it is normal, anti-tTG IgA antibody testing is ordered. To the contrary, anti-tTG IgG or anti-DGP IgG testing is requested. That strategy has not been validated in Mexico, nor is the rate of selective IgA deficiency known with certainty. On the other hand, many clinical laboratories simultaneously report anti-tTG IgA and IgG concentrations, and in others it is possible to order a test that combines anti-tTG IgA and anti-DGP IgG in the same sample. Cost-benefit analyses need to be conducted, considering the relatively low prevalence of CD in the Mexican population.55
Due to its popularity, many persons without an accurate diagnosis follow a gluten-free diet. That situation is increasingly more common and limits the usefulness of serologic markers. A practical measure is to perform a detailed dietary interview. It is not uncommon to find that persons inadvertently continue consuming gluten.2,40,55 In that case, autoantibody negativity can be real. In contrast, when no gluten is being consumed, the most recommendable strategy is to convince the patient to eat a normal diet, to then quantify autoantibodies.
17. In cases in which there is a high probability of CD, anti-tTG IgA/anti-DGP IgG, or anti-EMA antibody testing should be ordered from the very beginning.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 95%, in partial agreement 5%).
Even with laboratory tests that have high sensitivity and specificity (as is the case with autoantibodies for CD), positive predictive value and negative predictive value can be low, given that their performance depends on the prevalence of the disease being tested. If a statistical exercise is undertaken that takes into account the reported prevalence of CD in Mexico (0.7%) and the positive and negative predictive values are calculated considering the sensitivity and specificity of the anti-tTG IgA antibodies (90% and 95%, respectively), a positive anti-tTG IgA titer would have a positive predictive value of only 12%, signifying that the majority of the results (88%) would be false positives! In contrast, the same exercise applied to a population with a greater possibility a priori for CD, as occurs in groups identified as having high risk for the disease (see statements 4 and 5), would result in a positive predictive value close to 80%. Therefore, a recently published position article pointed out the limited usefulness of screening for CD in asymptomatic persons with no risk factors and of carrying out a targeted search for cases.56
The tactic proposed in statement 17 has not been validated, nor has it been proposed in other algorithms that follow the indications stated in the previous statements. However, it has a practical purpose: 1) the simultaneous quantification of anti-tTG IgA and anti-DGP IgG antibodies rules out the possibility that selective IgA deficiency could be producing false negatives; and 2) studies conducted on groups of patients with a high probability of presenting with CD have reported very high sensitivity and specificity figures (95% and 98%, respectively).2,40,55
The high specificity of anti-EMA antibodies has been widely demonstrated, signifying that a positive result practically confirms the presence of CD. However, the insufficient sensitivity of anti-EMA testing keeps it from being used as a screening test, given that a considerable number of celiacs have negative serum anti-EMA.55
18. Anti-tTG IgA and anti-EMA positivity supports CD diagnosis.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
The confirmation of CD diagnosis involves bringing together several elements that must necessarily arise form clinical suspicion. Serologic tests have become an indispensable tool, but if they are incorrectly interpreted, they can result in unjustified therapeutic measures being carried out. That situation is not rare, given that it is currently known that many persons following a gluten-free diet do not have a well-defined diagnosis. The problem is magnified in countries with a relatively low prevalence of CD, such as Mexico. Thus, it must be emphasized that diagnosis cannot be made from the positivity of a single test, but rather from a combination of other serologic assays.2,40,55 As if that were not enough, autoantibody titers must also be taken into account. For example, an isolated low concentration of anti-tTG IgA most certainly corresponds to a false positive value, but if it is accompanied by a positive anti-EMA value, then clinical suspicion is virtually confirmed.55
19. In adults, duodenal biopsy is essential to confirm CD diagnosis and establish the severity of damage to the mucosa.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
Different interested groups working in referral centers have reported on the excellent diagnostic yield of the autoantibodies. Sensitivity and specificity of both anti-tTG IgA antibodies and anti-EMA are much lower in the real world, in which the prevalence of CD is lower and other factors intervene, such as variations resulting from the use of different commercial kits, with respect to the suggested values for identifying celiacs, as well as the fact that the kits are not always optimal for that purpose. Thus, histologic confirmation through duodenal biopsy ensures the proper diagnosis, and should be carried out before indicating a costly, difficult, and limiting therapeutic measure, such as a lifetime gluten-free diet.2,35,40,57 Another benefit of histologic confirmation is that it enables the follow-up of patients following a restrictive diet that do not have an adequate response or continue to present with high autoantibody levels. The correct identification of refractory or treatment-resistant CD requires the demonstration of tissue damage in the small bowel mucosa. In addition, the persistence of intestinal villous atrophy has been shown, albeit with some controversy, to be correlated with a poorer outcome in celiacs.47
In 2012, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)58 proposed a diagnostic strategy for avoiding endoscopy in children that meet the following conditions: 1) 10-fold elevation of anti-tTG IgA above the cut-off value; 2) confirmation through a positive anti-EMA test from a different blood sample; 3) HLA DQ2/DQ8 positivity; and 4) compatible clinical symptoms (symptomatic CD). Those recommendations have been validated in retrospective analyses and prospective studies in both children and adults, as well as in populations with and without symptoms. However, they have not been universally accepted.
20. For a better evaluation of histologic alterations, at least 4 biopsy specimens should be taken from the distal duodenal mucosa and one or 2 from the duodenal bulb.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Histologic analysis requires at least 4 biopsies taken from the second part of the duodenum (D2). In a retrospective study, Pais et al.59 demonstrated the diagnostic superiority of 4 biopsies over 2 (100% vs. 90%). In a prospective cohort study, Kurien et al.60 reported a higher grade of villous atrophy in duodenal biopsies taken from the quadrant “between the 9 o’clock and 12 o’clock positions” (96.4% sensitivity; 95% CI: 79.7-100%) than from those taken “between the 12 o’clock and 3 o’clock positions” (92% vs. 65%, respectively; p=0.02).
In studies conducted in the United States and the United Kingdom, between 5% and 14% of the patients with a recent CD diagnosis were reported to have had a previous endoscopic evaluation. Therefore, to improve CD detection, recent studies have suggested taking biopsies from the first part of the duodenum (D1), increasing diagnostic yield by up to 10%,61 beside the fact that in some patients (1.8-14%) D1 is the only affected site (ultrashort CD).62-64 González et al.65 found D1 or D2 involvement in 90% of the cases and at both sites in 75% of the patients. However, it is relevant that CD diagnosis was only able to be made from histologic changes in D1 in 13% of the cases.
21. Histologic alterations should be evaluated and reported systematically through validated classifications.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
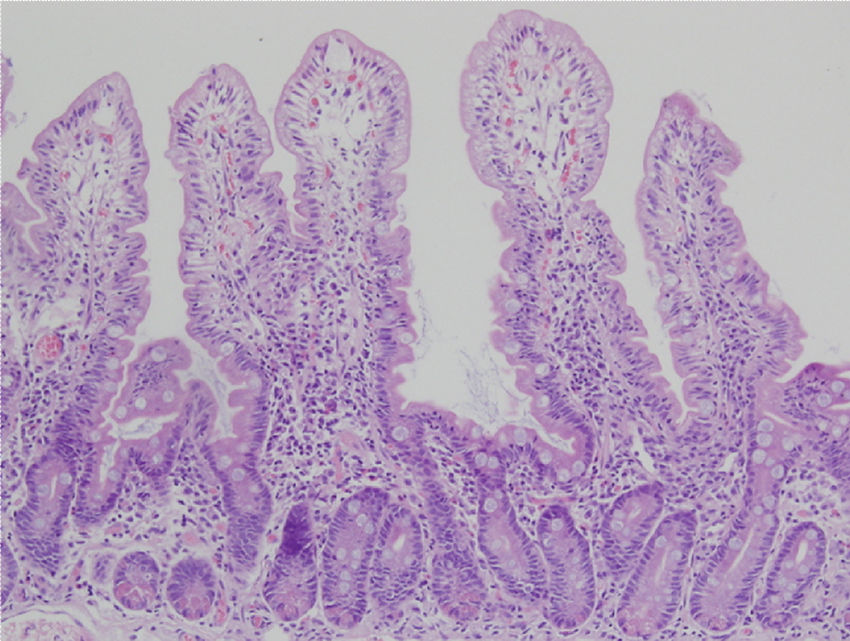
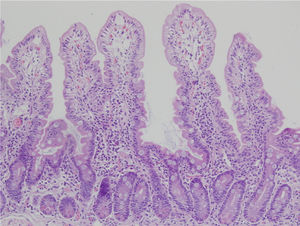
Biopsy samples can be fresh and fixed on paper or float freely in formalin.54-57 Histologic alterations should be evaluated in well-oriented biopsies, identifying at least 4 villi with the morphology shown in the photomicrograph in Figure 2. In that manner, the height of the villi and the depth of the crypts can be more accurately defined, enabling the villi/crypt ratio to be established, which is normally 3:1.57 The report should indicate the number of biopsies and those that are able to be evaluated, describing the following characteristics:
- •
Villi morphology, determining whether there is atrophy, and if so, if it is partial, subtotal, or total.
- •
Cellular content in the lamina propria (lymphocytes, plasma cells, eosinophils, or neutrophils).
- •
The presence of Brunner glands.
- •
The presence of crypt hyperplasia.
- •
The number of intraepithelial lymphocytes (IELs) for every 20 enterocytes in the tips of at least 5 villi, or for every 50 enterocytes. Fewer than 25 IELs for every 100 enterocytes is considered normal.2,57
- •
In borderline or equivocal counts, immunohistochemistry (CD3) can be useful in the quantification.
Duodenal biopsy for the histologic analysis of CD.
The histologic alterations should be evaluated in well-oriented biopsies, identifying at least 4 villi that have the morphology shown in the photomicrograph. The description should include: a) morphology of the villi, the presence or absence of atrophy, and whether atrophy is partial, subtotal, or total; b) cellular content in the lamina propria (lymphocytes, plasma cells, eosinophils, or neutrophils); c) presence of Brunner glands and crypt hyperplasia; and d) number of intraepithelial lymphocytes, with fewer than 25 lymphocytes/100 enterocytes considered normal.
22. If there is intestinal villi atrophy with negative autoantibodies for CD, other diseases should be ruled out.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
In our medical environment, it is not unusual to find persons diagnosed with CD based only on histopathologic findings. Increased IELs, crypt hyperplasia, and villous atrophy can be found in other conditions that are not rare in Mexico, such as giardiasis, bacterial overgrowth, the use of certain medicines (olmesartan, nonsteroidal anti-inflammatory drugs), tropical sprue, soy protein allergy, and milk allergy.66-68 Those same histologic alterations can also be observed in less common diseases, such as autoimmune enteropathy, host-versus-graft disease, and inflammatory bowel disease, and they should be included in the differential diagnosis.69
23. DQ2/DQ8 histocompatibility antigen analysis is useful for ruling out CD in special situations, such as villous atrophy with negative serology (discrepancy between serology and histology) and/or in persons that are following a gluten-free diet and refuse to undergo a challenge test.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
The expression of class II major histocompatibility complex antigens: HLA DQ2 (encoded by the A1*05 and B1*02 alleles) or HLA DQ8 (encoded by the A1*03 and B1*0302 alleles) is essential for CD to develop. Therefore, when serologic tests are negative, or a gluten challenge cannot be performed, testing for those alleles is of diagnostic help.40,70-72 Their absence practically rules out the disease (high negative predictive value> 99%), making it necessary to search for other causes that explain the symptoms or histopathologic alterations. Nevertheless, their presence does not confirm the disease, given that 30-40% of the populations in Europe, Asia, and America, including Mexico (24% DQ8 and 16% DQ2), express those haplotypes or their alleles, but only 3% of them have CD (low positive predictive value < 12%), making them a necessary, but clearly insufficient, feature for developing CD.73
The analysis of the HLA molecules is also useful for ruling out CD, requiring additional screening to be carried out in persons at high risk, such as first-degree relatives of celiacs (see statements 4 and 5). It should be emphasized that routine genetic testing should not be performed diagnostically in patients suspected of presenting with CD. In the Mexican Mestizo population, CD is mainly associated with the HLA- DQ8 genotype.16
24. Enteroscopy and video capsule (VC) endoscopy studies are justified only in cases of complicated CD, in which premalignant or malignant lesions of the small bowel are suspected.
Quality of evidence and strength of recommendation: B2 weak in favor of the statement (in complete agreement 100%).
Endoscopy has become an indispensable tool in the diagnosis of CD, given that despite its low sensitivity for detecting villous atrophy, duodenal biopsies can be taken, which continue to be the gold standard for diagnosis.74
Due to its operational characteristics (safe and minimally invasive), the use of VC endoscopy is an area in continuous development. In a study conducted on celiacs with diagnosis confirmed through duodenal biopsies, it was found that the sensitivity for identifying villous atrophy was significantly higher with VC endoscopy than with conventional endoscopy (92% vs. 55%, p=0.0005). Specificity was 100% with both methods, with involvement of the duodenal mucosa in more than 90% of the cases (duodenum in 32% and duodenum/jejunum in 59%). In addition, VC endoscopy enabled disease extension and treatment response to be evaluated.75
VC endoscopy can be useful in other well-selected cases. For example: in persons with negative serology and minimal histologic changes (Marsh I-II) that have rejected endoscopic study or are contraindicated for it; in persons with endoscopic changes suggestive of atrophy, with negative biopsy, and elevated diagnostic suspicion; or in cases with lesions in patches (15%), and thus susceptible to sampling error in the duodenal biopsies.76-78 However, its use as the only diagnostic method is limited, due to the fact that the findings are not pathognomonic and that, at present, it is not possible to obtain a sample for histologic confirmation.
VC endoscopy has been shown to be useful in cases of refractory CD with adequate concordance with histology in cases of atrophy, as well as in the evaluation of disease extension (greater extension in cases of type II refractory CD, compared with type I refractory CD, 54% vs. 9%) and factors associated with poor outcome.79-80 Recently, the roles of VC endoscopy and enteroscopy for detecting premalignant and malignant lesions in complicated CD cases were assessed and a general overall yield close to 20% was found, which has implications for the treatment of that group of patients, due to the prognosis associated with the nature of those lesions.81 In addition, VC endoscopy aids in evaluating treatment response. Duodenal biopsies do not reflect the degree of improvement because the changes in the cicatrization of the mucosa are produced in a distal to proximal direction.82
Due to the above, and in accordance with the evidence in the medical literature, the use of VC endoscopy and enteroscopy is justified only in cases of refractory CD or in those patients with adequate treatment response in whom symptoms reappear or persist.
25. The CD diagnostic strategy in persons that are following a gluten-free diet includes a gluten challenge with 3g of gluten/day for 4-6 weeks, determining anti-tTG IgA antibodies.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
The great popularity of the gluten-free diet has resulted in an ever-increasing number of persons designating themselves as celiacs and establishing CD diagnosis in those persons is a challenge. A simple approximation would consist of knowing their HLA status. If none of the haplotypes that are risk factors (DQ2/DQ8) are expressed, then the disease is ruled out. But if they present with one of the alleles, which is expected a priori in 40% of the cases, then a gluten challenge to quantify anti-tTG IgA should be proposed. It has recently been demonstrated that the ingestion of low doses of gluten (3g/day) during relatively short periods (2 weeks) is sufficient for inducing a serologic response and compatible histologic changes. Nevertheless, due to the fact that antibody production can be slow in some cases, several experts suggest prolonging the diet 6 weeks or more if clinical conditions allow it.83 That dose can be reached through the regular consumption of at least 2 slices of bread for the established period of time.
26. CD is ruled out in a person with symptoms associated with gluten ingestion that has negative serology, normal histology, and negative HLA DQ2/DQ8 haplotypes.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
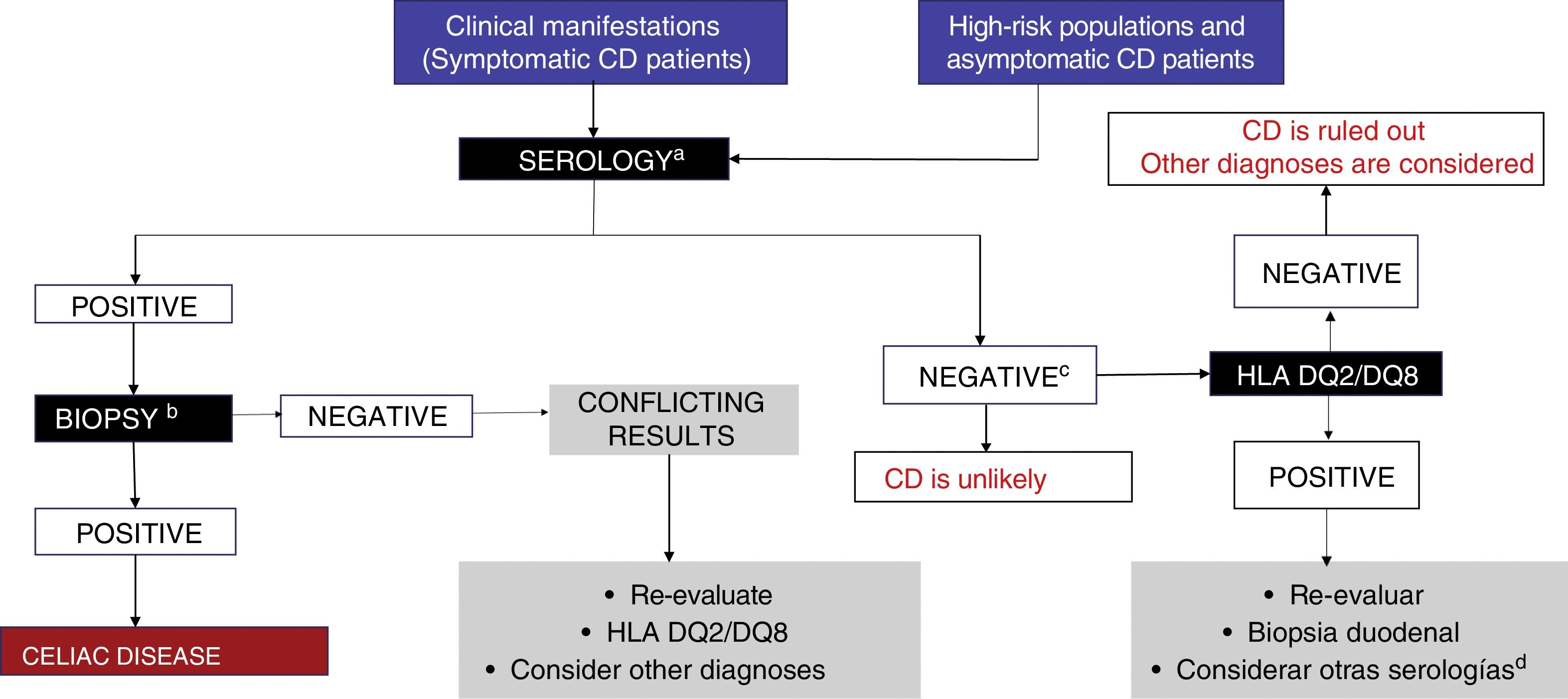
CD is diagnosed through positive serologic tests and compatible biopsy, and in the event of discrepancy, class II HLA allele testing is carried out (fig. 2). If those tests are negative, then alternative causes explaining the symptoms should be searched for, such as non-celiac gluten (wheat) sensitivity, gluten allergy, or fermentable short-chain carbohydrate intolerance. To do so requires that special tests, such as the double-blind gluten/placebo challenge should be carried out to evaluate symptom reproducibility.13 Those patients should be referred to centers with interest and experience in the area of CD.2,13,40
Figure 3 illustrates the diagnostic algorithm for CD in Mexico proposed by the group of experts.
Diagnostic algorithm.
a IgA, tTG-IgA and DGP-IgG testing is recommended while subjects are receiving a diet with gluten.
b At least 4 biopsies from the second part of the duodenum and one-to-2 from the duodenal bulb.
C The decision to perform genetic testing should be individualized (it is unnecessary in the majority of patients).
d Consider anti-EMA testing.
EMA: endomysial antibody; DGP: deamidated gliadin peptide; tTG: tissue transglutaminase.
27. A gluten-free diet (GFD) is essential for the treatment of CD. Patients with CD must adhere to a lifetime gluten-free diet.
Quality of evidence and strength of recommendation: A1 strong in favor of the statement (in complete agreement 100%).
The GFD is absolutely essential for the patient with CD because there is currently no medicine that prevents damage to the intestinal mucosa resulting from exposure to gluten. That diet reduces morbidity and mortality, improves osteopenia, osteoporosis,84 anemia,85 the risk for malignant diseases,86 and gastrointestinal symptoms,87 as well as greatly improving the quality of life of patients with CD.88 That is achieved by not consuming wheat or its hybrids (triticale, spelt, kamut), barley, rye, and ingredients derived from them, or foods that could be contaminated by those grains or their derivatives.89 Patients with CD must adopt the GFD for the rest of their lives.
28. The consumption of foods with less than 20ppm of gluten is considered safe for patients with CD.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Even though between 20 and 100ppm of gluten in foods is considered a tolerable quantity, according to the Codex Alimentarius, FDA, and EFSA, less than 20ppm of gluten in foods or food products is the quantity required to be considered safe for patients with CD, 6,90 classifying such foods as “gluten-free”.91
Some agencies, such as the “Gluten-Free Certification Organization” certify foods containing less than 10ppm.92 In Mexico there are associations of celiacs such as ACELMEX (www.acelmex.org.mx) and Celíacos de México (www.celiacosdemexico.org.mx), among others, that have complete information with respect to the content of gluten in foods. If there is doubt as to the presence or amount of gluten in a food, it is better not to eat it.
29. A lactose-free diet is recommendable for the patient recently diagnosed with CD, for a varying period of time, depending on tolerance. Oatmeal that is not contaminated with gluten can be introduced after having maintained a GFD for 3-6 months.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 82%, in partial agreement 18%).
Patients with CD are likely to initially present with malabsorption and lactose intolerance secondary to intestinal villous atrophy.93 To diminish symptoms while the atrophy is being reverted, a lactose-free diet is recommendable.94 According to the ESPGHAN guidelines, a routine lactose-free diet is not recommended for children, and if it is necessary, should be indicated for short periods.58
Patients with CD can gradually include oatmeal that is not contaminated with gluten, at a quantity of 50g (dry weight) daily, which is safe to consume and makes following the GFD easier, by offering one more food option to patients.95
30. All patients with CD should be evaluated by a nutritionist with experience in the management of the disease.
Quality of evidence and strength of the recommendation: A1 strong in favor of the statement (in complete agreement 100%).
Multidisciplinary care is indispensable in the comprehensive treatment of patients with CD. The American Dietetic Association recommends that nutritional therapy be guided and supervised by a certified nutritionist, coordinating the nutritional care with the rest of the team of clinical professionals.96
31. The initial evaluation of the patient diagnosed with CD should include the quantification of levels of glucose, serum electrolytes, iron, vitamin B12, and vitamin D, and the performance of a liver panel, complete blood count, and bone densitometry, to determine the impact of the disease on the patient's nutritional status.
Quality of evidence and strength of recommendation: B2 weak in favor of the statement (in complete agreement 82%, in partial agreement 18%).
Because it is likely that at the time of CD diagnosis villous atrophy has conditioned vitamin malabsorption, metabolic alterations, and malnutrition, it is essential to evaluate the nutritional status of the patient through biochemical analyses.1-2,40,58 Thus, the following tests should be ordered for all patients with CD, regardless of clinical signs:
- •
Glucose and serum electrolytes.
- •
Lipid profile.
- •
Liver function tests.
- •
Complete blood count, iron profile, folic acid, and vitamin B12.
- •
25-hydroxy vitamin D.
- •
Bone densitometry.
In the case of patients with CD and iron-deficiency anemia, daily consumption of gluten-free multivitamins with iron or an additional individually calculated therapeutic dose of iron are recommended. According to the American Dietetic Association, iron supplementation and its follow-up should be repeated until normal hemoglobin values are reached.96 The recommendation is similar for patients with folic acid and vitamin B12 deficiencies.94
Baseline bone densitometry is supported by reports that at the time of diagnosis, patients with CD present with important mineral and bone mass loss. Densitometry should be repeated according to the established criteria for the detection and management of osteopenia and osteoporosis.96-98
32. Strict adherence to the diet should be evaluated by the treating group (physician and nutritionist) at the office visits.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
The aim of periodic evaluation by the multidisciplinary team is to evaluate the alimentation plans and provide patients with recommendations for preventing inadvertent gluten exposure or cross-contamination.1-2,97 It is advisable to share information sources and educate celiacs to read the ingredients listed on the labels of food products and supplements, thus preventing inadvertent gluten ingestion.
33. Adult patients with CD must make sure to consume at least 1,000mg calcium/day. Pediatric patients must be sure to consume the recommended daily amount.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Because patients with CD can present with osteopenia/osteoporosis, calcium and other mineral supplementation is recommendable, but only in those patients that do not receive the daily requirements by age (Table 4)98 from a varied diet.98-101 Supplements should contain vitamin D and be gluten-free.
Calcium requirements according to age.
| Age | mg/day |
|---|---|
| 0-6 months | 210 |
| 7- 12 months | 270 |
| 1- 3 years | 500 |
| 4- 8 years | 800 |
| 9- 18 years | 1,300 |
Adapted from: Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes.98
34. The aim of follow-up is to evaluate symptom improvement and identify the appearance of complications. Strict follow-up of growth and development is essential in children with CD.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Treatment and surveillance of patients with CD is life-long. Although there is little evidence with respect to the most effective means of surveillance, periodic evaluation by the physician and nutritionist is recommended for symptom resolution control, growth maintenance and continued development (in the pediatric population), dietary review, and repeat serologic tests. During those evaluations, the health professional should reinforce the benefits of carrying out a strict lifetime gluten-free diet.97,102
35. Follow-up in patients with CD should include anti-tTG IgA antibody testing at least every 6 months the first year.
Quality of evidence and strength of recommendation: B2 weak in favor of the statement (in complete agreement 95%, in partial agreement 5%).
GFD compliance is established through a combination of symptoms and serology (anti-tTG IgA or anti-DGP IgA or IgG). Serologic markers depend on gluten consumption and their values decrease or increase accordingly. They can become negative after a few months on the GFD or their titers increase or become positive when a gluten challenge is carried out.97,103-104 In children, negativization of the anti-tTG IgA and anti-DGP IgG antibodies is a sensitive and specific marker of recovery of the mucosa.94 In adults, recovery of the mucosa is slower and serologic marker negativization is not correlated with normalization of the atrophy of the intestinal mucosa.105 In addition, because the markers are not useful for detecting very low levels of ingested gluten, tests have been developed that quantify immunogenic peptide excretion of gluten in urine or stool, thus detecting recent gluten consumption.106-107
36. When the patient with CD does not respond to treatment or there are relapses, compliance with the dietary recommendations should be thoroughly reviewed and the possibility of cross-contamination or inadvertent gluten ingestion should be ruled out. Other causes, such as medication use, infectious or inflammatory processes, or functional disorders should also be ruled out.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
In CD that does not respond to treatment, the patient persists with signs, symptoms, or laboratory test alterations typical of CD, despite consuming a GFD for 6 to 12 months. It occurs in 10-30% of patients with CD.45 The most frequent causes are: inadvertent gluten exposure (36%), irritable bowel syndrome (22%), refractory CD (10%), lactose intolerance (8%), and microscopic colitis (6%).107 In a study on 56 Mexican patients with CD, inadvertent gluten ingestion presented in 34%.108
In some cases, symptom persistence may be due to milk casein or corn zein.109 Nevertheless, both milk and corn are considered safe foods for the majority of celiacs.
37. When the patient does not respond to a strict diet, and other causes have been ruled out, upper gastrointestinal endoscopy with biopsy is recommended.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Intestinal biopsy is the only method for documenting the normalization of the intestinal mucosa.97,104 In adults, complete recovery of the mucosa after beginning a GFD can last up to 3 years,110 whereas in children, that same recovery is reached in fewer than 2 years.111 If follow-up intestinal biopsies are normal, patient symptoms may be due to other causes. If the biopsies continue to show atrophy and/or lymphocytic infiltration, then inadvertent gluten exposure, bacterial overgrowth, or other causes of atrophy (e.g., nonsteroidal anti-inflammatory drugs or other medications), and finally, refractory CD, should be considered.112 To make the diagnosis of that last pathology, specific immunohistochemical and molecular studies for investigating type II refractory CD should be ordered.113
38. Patients with refractory CD should be referred to centers with experience in that condition.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Even though refractory CD is rare, those patients present with severe malnutrition and risk of lymphoma and sepsis, and their evaluation at specialized centers is recommended.1,57,114 Even though there are no controlled clinical trials on the theme, drugs such as budesonide, prednisone, and/or immunosuppressants can be used.115-118 In patients that develop lymphoma, surgery, chemotherapy, and even bone marrow transplantation can be attempted, albeit they have shown very limited efficacy.119
39. A strict, lifetime GFD is only recommended in patients with CD.
Quality of evidence and strength of recommendation: B1 strong in favor of the statement (in complete agreement 100%).
Because the GFD is restrictive, with little diversity, and the gluten-free industrialized products are expensive, it is recommended only in patients with CD and wheat allergy.1,2,40,108 Lifetime GFD in patients with non-celiac gluten sensitivity is a subject of debate.89
Furthermore, despite the enormous increase of gluten-free products in the market, not ingesting wheat derivatives has not been shown to have health benefits in the general population.89 Gluten-free products are known to contain less protein (up to 70% less), especially pastas and breads.120 A GFD can be associated with an increase in body mass and total cholesterol and a decrease in triglycerides and homocysteine.121 In a recent study on the general population, a diet low in gluten was not shown to have any beneficial effects on cardiovascular risk, and when adjusted to whole-grain cereal consumption, gluten ingestion had a protective effect.122 Finally, the GFD is difficult to implement and up to 3 times more expensive (> 500% for bread) than a conventional diet.123
ConclusionsThe present guidelines emphasize that epidemiology and risk factors associated with CD (first-degree relatives, autoimmune diseases, high-risk populations) are similar in Mexico to those described in other parts of the world. They establish standards for the appropriate diagnosis and multidisciplinary management of Mexican patients with CD. The fact that a gluten-free diet is indispensable only in persons with confirmed CD is underlined, as well as the ongoing controversy about the role of the strict diet in gluten-sensitive non-celiac patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureThe present consensus was carried out with the economic support of the Asociación Mexicana de Gastroenterología, which made the participation, transportation, and accommodations during the face-to-face meeting possible.
Conflict of interestDr. Luis F Uscanga-Domínguez is a Member of the Advisory Board of Asofarma.
Dr. José María Remes-Troche is a Member of the Advisory Board of Takeda Pharmaceuticals, Alfa-Wassermann, and Asofarma. He has received research funds from Sanfer and is a Speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Almirall, and Astra-Zeneca.
Dr. Alberto Rubio-Tapia declares that he has no conflict of interest.
Dr. Guillermo Rafael Aceves Tavares declares that he has no conflict of interest.
Dr. Ana María Calderón de la Barca declares that she has no conflict of interest.
Dr. Ramón Carmona Sánchez is a Member of the Advisory Board of Mayoly-Spindler, a Speaker for Mayoly-Spindler and Grünenthal, and participates in research protocols funded by Laboratorios Senosian and Asofarma.
Dr. Eduardo Cerda Contreras declares that he has no conflict of interest.
Dr. Enrique Coss-Adame is a Member of the Advisory Board of Takeda Pharmaceuticals aand Carnot Laboratories and is a Speaker for Takeda, Asofarma, Alfa-Wassermann, and Carnot.
Dr. María Eugenia Icaza Chávez is a Speaker for Asofarma, Takeda, and Astra-Zeneca.
Dr. Aurelio López-Colombo is a Speaker for Takeda and Alfa-Wassermann.
Dr. María del Pilar Milke-García declares that she has no conflict of interest.
Dr. Miguel Morales Arámbula is a Speaker for Takeda and Grünenthal.
Dr. Mario Peláez-Luna declares that he has no conflict of interest.
Dr. París Ramos Martínez declares that he has no conflict of interest.
Dr. Sergio Sánchez Sosa declares that he has no conflict of interest.
Nutritionist María Cristina Treviño Mejía declares that she has no conflict of interest.
Dr. Rodrigo Vázquez Frías is a Speaker for Nestlé and Sanofi.
Dr. Liliana Beatriz Worona Dibner declares that she has no conflict of interest.
Dr. Luis Eduardo Zamora-Nava declares that he has no conflict of interest.
Please cite this article as: Remes-Troche JM, Uscanga-Domínguez LF, Aceves-Tavares RG, Carderón de la Barca AM, Carmona-Sánchez RI, Cerda-Contreras E, et al. Guía clínica para diagnóstico y tratamiento de la enfermedad celíaca en México. Revista de Gastroenterología de México. 2018;83:434–450.