Follicular gastritis is associated with Helicobacter pylori infection, but little is known of its relation to bacterial genotypes. Our aim was to establish the relation between follicular gastritis and different H. pylori strains.
Materials and methodsAn analytic case-control study was conducted that included 36 patients with follicular gastritis (cases) and 83 with nonatrophic gastritis (controls). The sociodemographic information was obtained through a questionnaire. Biopsies were evaluated according to the Sydney System and the Wotherspoon scoring system. Helicobacter pylori genotyping was performed using the polymerase chain reaction technique. The quantitative variables were presented as mean and standard deviation and the qualitative variables as proportions and absolute frequency. The effect of each variable on outcome (follicular gastritis) was evaluated through the odds ratio and its 95% confidence interval. Statistical significance was set at a p<0.05.
ResultsFollicular gastritis was associated with Helicobacter pylori infection (OR: 13.41, CI: 1.7-103, p=0.01). The CagA+ genotype was present in 56.5% of the cases and 58% of the controls. The cytotoxic VacAs1m1strain was present in 82% of the isolates in both groups. IceA1 frequency was 34.8% in the cases and 26% in the controls and the difference was not statistically significant.
ConclusionsThe population studied had elevated frequencies of cytotoxic Helicobacter pylori strains and the iceA1 genotype was more frequent in follicular gastritis.
La gastritis folicular se asocia con la infección por Helicobacter pylori; sin embargo, su relación con los genotipos bacterianos es poco conocida. Nuestro objetivo fue establecer la relación entre la gastritis folicular y distintas cepas del H. pylori.
Material y métodosEstudio analítico de casos y controles que incluyó 36 pacientes con gastritis folicular (casos) y 83 con gastritis no atrófica (controles). La información sociodemográfica se obtuvo mediante un cuestionario. Se obtuvieron biopsias que se evaluaron según las clasificaciones de Sydney y Wotherspoon. La genotipificación de H. pylori se realizó mediante reacción en cadena de la polimerasa. Para las variables cuantitativas se empleó la media y la desviación estándar. Las variables categóricas se presentan como proporciones y frecuencia absoluta. La OR con su IC del 95% evaluó el efecto de cada variable sobre el desenlace (gastritis folicular). Un valor de p<0.05 se consideró significativo.
ResultadosLa gastritis folicular se asoció con la infección por Helicobacter OR: 13.41 IC (1.7-103) p=0.01. El genotipo CagA+ estuvo presente en el 56.5% de los casos y 58% de los controles. El 82% de los aislamientos presentó la cepa citotóxica VacAs1m1 en los 2 grupos. La frecuencia de iceA1 fue de 34.8% y 26% en casos y controles respectivamente, diferencias que no fueron estadísticamente significativas.
ConclusionesLas cepas citotóxicas de Helicobacter exhiben elevadas frecuencias en la población estudiada. El genotipo iceA1 fue más frecuente en la gastritis folicular.
Follicular gastritis is characterized by a marked mononuclear inflammatory infiltrate and the formation of lymphoid follicles with a germinal center in the lamina propria.1,2 For some authors, at least 2 secondary lymphoid follicles should be identified in an area 1cm from the gastric mucosa to make the diagnosis.3 Disease prevalence is variable. In Japan a prevalence of 0.19% was reported, Greek and French researchers described a prevalence of 12.7% and 14.2%, respectively,4,5 and in other studies, prevalence varied from 27.4% to 79.9%.6–8 A Colombian study described prevalence at 8.4%.9
The disease is associated with age, Helicobacter pylori (H. pylori) infection, and the development of mucosa-associated lymphoid tissue (MALT) lymphoma.1,10 In relation to age, a greater frequency of the disease has been determined in pediatric patients with persistent dyspeptic symptoms, and in adolescents and young adults. Female sex appears to be a special condition for the development of the pathology.5,11 Of greater importance is the relation of follicular gastritis to MALT lymphoma. Even though lymphoma etiology can be linked to multiple factors, Helicobacter infection appears to play a dominant role. That role has been demonstrated, following the regression of follicular gastritis and even MALT lymphoma after bacterial eradication.12 Nevertheless, little is known about the cascade of events that occurs from follicular gastritis to the appearance of lymphoma.13
The relation of bacterial genotypes to certain kinds of diseases is well-known. The cagA+and vacAs1/m1 genotypes are considered the most virulent strains associated with peptic ulcer, gastric cancer, and severe inflammation,14 whereas the s2/m2 strain produces limited inflammation. However, little is known about the role of the bacterial genotypes in the origin of follicular gastritis. In that context, the aim of the present study was to establish the relation between the genetic variability of H. pylori (genotypes cagA, vacA, and iceA1) and follicular gastritis. The intention was to broaden the understanding of the determining factors of that condition and establish whether there is a bacterial genotype associated with it. Because follicular gastritis is an entity related to gastric lymphoma, the hypothesis is that the most aggressive bacterial genotypes are associated with that type of inflammation.
Materials and methodsAn analytic unmatched case-control study was conducted on patients that were admitted to the gastroenterology units of the Hospital Universitario San José and Endovideo in the city of Popayán (located in Southwest Colombia in the Andes and catalogued as a high-risk area for gastric cancer),15 within the time frame of January 2008 and December 2011. The study sample was obtained through convenience sampling and included patients above 18 years of age, with a histopathologic diagnosis of follicular gastritis or nonatrophic gastritis (the control group). The participants, as well as their parents, had to be born in a municipality of the Cauca department. Patients with atrophy, metaplasia, gastric cancer or other type of cancer, gastric surgery, previous treatment for H. pylori, or HIV infection were excluded.
Biologists from the Grupo de Investigación en Genética Humana y Aplicada (GIGHA) at the Universidad del Cauca applied a questionnaire to collect the sociodemographic variables (age, sex, educational level, income, and ethnic group). Gastric samples were obtained through gastrointestinal endoscopy performed by experienced gastroenterologists. Patients were referred for endoscopy due to dyspeptic symptoms and they arrived for the examination having fasted for at least 8hours. The participants were not sedated but received a topical solution to anesthetize the pharynx. Five samples were taken: 2 biopsies from the antrum (the greater and lesser curvatures), 2 from the corpus (the greater and lesser curvatures) and one from the incisura angularis. The biopsies were fixed in buffered formalin and stained with the hematoxylin-eosin and Giemsa stains. The histopathologic analysis of each sample was carried out by 2 pathologists. Visual analogue scales were employed according to the Sydney system. The presence of both histologic activity (inflammation due to polymorphonuclear neutrophils) and H. pylori was evaluated. Follicular gastritis was defined as the presence of one or more lymphoid follicles with germinal centers in the lamina propria, and its severity was staged on a scale from 1 to 5, according to the Wotherspoon system16 (Table 1).
Follicular gastritis severity grade, including the suspicious MALT lymphoma grade.
| Grade 0 | Normal | Scant inflammatory cells in the lamina propria. No lymphoid follicles |
| Grade 1 | Chronic gastritis | Small clusters of lymphocytes in the lamina propria. No lymphoid follicles |
| Grade 2 | Follicular gastritis | Prominent lymphoid follicles with plasma cells and mantle zone |
| Grade 3 | Suspicious, probably reactive, inflammatory infiltrate | Lymphoid follicles surrounded by small lymphocytes that diffusely infiltrate the lamina propria and occasionally the epithelium |
| Grade 4 | Suspicious infiltrate, probably lymphoma | Lymphoid follicles surrounded by centrocyte-like cells that infiltrate the lamina propria and the epithelium in small groups |
| Grade 5 | MALT lymphoma | Presence of a dense infiltrate of centrocyte-like mononuclear cells in the lamina propria that invade the epithelial lamina—lymphoepithelial lesion— |
Source: Modified from Wotherspoon et al.16
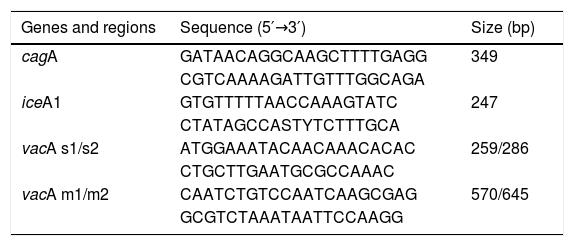
To carry out the bacterial genotyping studies, DNA was extracted from the biopsies embedded in paraffin, utilizing the Chelex technique (No. C7901; Sigma, St. Louis, MO). The cagA, iceA1, and vacA genes were amplified using the polymerase chain reaction (PCR) method previously described by Yamaoka et al.12 The PCR mixtures were prepared with 50 ng of genomic DNA, 100μmol dNTPs, 2.5μl of 10X PCR buffer, 1.0mM MgCl2, 1 U of Taq DNA polymerase (No. M1665; Promega, Madison, WI), and 30pmol of each of the primers, as indicated in Table 2. The reactions began with a denaturalization step at 95°C for 1minute, followed by 35 denaturalization cycles at 94°C for 1minute, hybridization at 52°C for 1minute and extension at 72°C for 1minute, and a final extension step at 72°C for 10minutes. The products were analyzed through electrophoresis in 2% agarose gels at 80 volts for 40minutes and the genotypes were recognized according to the expected size of the base pairs.
Sequence of primers for amplification through PCR.
| Genes and regions | Sequence (5′→3′) | Size (bp) |
|---|---|---|
| cagA | GATAACAGGCAAGCTTTTGAGG | 349 |
| CGTCAAAAGATTGTTTGGCAGA | ||
| iceA1 | GTGTTTTTAACCAAAGTATC | 247 |
| CTATAGCCASTYTCTTTGCA | ||
| vacA s1/s2 | ATGGAAATACAACAAACACAC | 259/286 |
| CTGCTTGAATGCGCCAAAC | ||
| vacA m1/m2 | CAATCTGTCCAATCAAGCGAG | 570/645 |
| GCGTCTAAATAATTCCAAGG |
The NCTC-11637 and NCTC-11638 H. pylori strains and the clinical 3062 isolate were provided by the Instituto Nacional de Cancerología de Colombia to be used as positive controls. In addition, all the PCR tests had internal amplification controls and adequate molecular markers (ladders).
Demographic information biases were controlled through a questionnaire with closed questions applied by members of the GIGHA, who received instructions and guidance from gastroenterologists and pathologists. Histopathologic evaluation was validated by a second pathologist.
The molecular biology tests were performed according to worldwide accepted protocols. Pilot tests were carried out to verify the quality of the reagents and the extraction kits and the laboratory equipment was previously calibrated. Cases of bacterial coinfection determined through the presence of more than one genotype were excluded from the analysis to prevent interpretation bias of the genetic information and to calculate the true effect of each genotype on the response variable.
Because the variable of age had normal distribution through the Kolmogorov-Smirnov test, it was presented as mean and standard deviation. The differences between the means were evaluated using the Student's t test, whereas the differences in proportions were evaluated through the chi-square test of independence. The OR and its p value were used to evaluate the effect of each study variable on the response variable (follicular gastritis). A multivariate logistic regression analysis was carried out, with sex and age as possible confounders. Statistical significance was set at a p<0.05. The statistical analysis was performed using the SPSS version 23 program.
The participants agreed to partake in the study and signed statements of informed consent. The study was approved by the Ethics Committee for Scientific Research of the Universidad del Cauca.
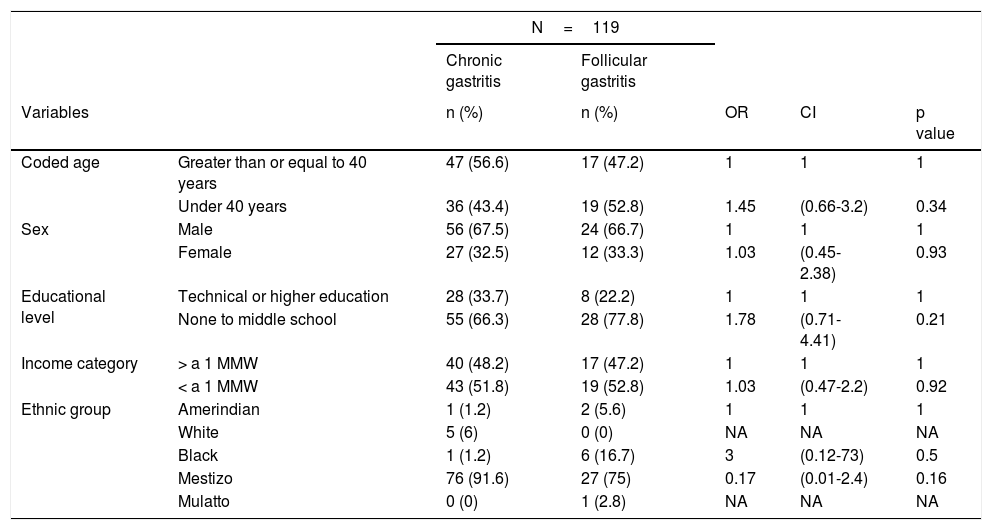
ResultsFrom a total of 808 participants selected within the study time frame, 119 patients met the inclusion criteria. Of those patients, 83 corresponded to the 70% that had nonatrophic gastritis and 36 to the 30% diagnosed with follicular gastritis. The age range in the patients with nonatrophic gastritis was 19 to 81 years, with a mean of 44.11 years. The age range in the patients with follicular gastritis was 21 to 82 years, with a mean of 41.86 years. The Student's t test showed no significant differences in the comparison of age means (p=0.48). In relation to ethnicity, the large majority of patients were mestizos, but there were 6 (17%) black patients in the follicular gastritis group. No statistically significant differences in the sociodemographic variables were found (Table 3).
Sociodemographic characteristics of the patients.
| N=119 | ||||||
|---|---|---|---|---|---|---|
| Chronic gastritis | Follicular gastritis | |||||
| Variables | n (%) | n (%) | OR | CI | p value | |
| Coded age | Greater than or equal to 40 years | 47 (56.6) | 17 (47.2) | 1 | 1 | 1 |
| Under 40 years | 36 (43.4) | 19 (52.8) | 1.45 | (0.66-3.2) | 0.34 | |
| Sex | Male | 56 (67.5) | 24 (66.7) | 1 | 1 | 1 |
| Female | 27 (32.5) | 12 (33.3) | 1.03 | (0.45-2.38) | 0.93 | |
| Educational level | Technical or higher education | 28 (33.7) | 8 (22.2) | 1 | 1 | 1 |
| None to middle school | 55 (66.3) | 28 (77.8) | 1.78 | (0.71-4.41) | 0.21 | |
| Income category | > a 1 MMW | 40 (48.2) | 17 (47.2) | 1 | 1 | 1 |
| < a 1 MMW | 43 (51.8) | 19 (52.8) | 1.03 | (0.47-2.2) | 0.92 | |
| Ethnic group | Amerindian | 1 (1.2) | 2 (5.6) | 1 | 1 | 1 |
| White | 5 (6) | 0 (0) | NA | NA | NA | |
| Black | 1 (1.2) | 6 (16.7) | 3 | (0.12-73) | 0.5 | |
| Mestizo | 76 (91.6) | 27 (75) | 0.17 | (0.01-2.4) | 0.16 | |
| Mulatto | 0 (0) | 1 (2.8) | NA | NA | NA | |
MMW: monthly minimum wage; NA: not applicable.
P values < 0.05 are statistically significant.
The relation between H. pylori infection and the follicular inflammatory response was evaluated in the histopathologic study. Thirty-five patients (97.2%) in the follicular gastritis group had the infection, whereas only 60 (72.3%) patients in the control group presented with it. There was a statistically significant association between infection and follicular gastritis; OR: 13.41, IC (1.7-103) p=0.01.
A statistically significant association also was found in the evaluated relation between bacterial infection and inflammatory activity (OR: 337.33, IC: [53.1-2142], p=0.00).
Follicular gastritis was classified as Wotherspoon grade 2 gastritis in the majority of the case group patients (23/36), as grade 3 gastritis in 12 of the case patients (33%), and as grade 4 gastritis in only one case patient. By sex, 6 men and 17 women had grade 2 gastritis, 6 men and 6 women presented with grade 3, and one woman, 42 years old, had grade 4. There were no documented cases of MALT lymphoma.
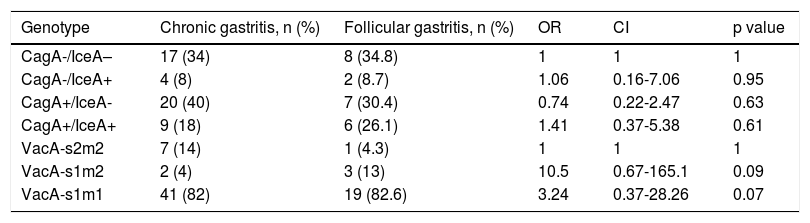
Of the total of 119 participants, 46 patients were excluded from the genetic analysis: more than one vacA bacterial genotype (coinfection) was found in 20 patients and gene amplification was not achieved in 26 patients (Table 4).
Through molecular testing, the H. pylori of 73 participants (50 patients with nonatrophic gastritis and 23 patients with follicular gastritis) was characterized. Regarding the distribution of the strains, 29 (58%) patients in the control group were positive for the cagA genotype, whereas in the follicular gastritis group that genotype was described in 13 (56.5%) patients. No statistically significant differences were found in relation to the cagA genotype (the OR for developing follicular gastritis in cagA+patients was 0.79, CI=0.29-2.13, and p=0.64).
In the patients with follicular gastritis, 8 (34.8%) were iceA1 positive, whereas in the control group that genotype was found in 13 (26%) patients. Those differences were not statistically significant (OR: 1.83, CI: 0.64-5.22, p=0.26). The s1, s2, m1, and m2 subtypes were determined in the vacA bacterial genotype analysis and they were grouped into 4 categories, according to their virulent profile. Those groups were then related to the other genotypes (iceA1 and cagA). The cagA–/iceA1– genotypes and the vacA s2/m2 subtypes were selected as contrast groups. The analysis showed a high prevalence (82%) of the vacA s1/m1 subtypes for the 2 patient groups with no significant differences (OR: 1.04, CI: 0.28-3.81, p=0.95).
The s2/m1 subtypes were not documented in either of the 2 groups and the analysis of the rest of the subtypes produced no statistically significant differences (Table 5).
Distribution of the cagA, iceA1, and vacA genotypes in the study groups; n=73.
| Genotype | Chronic gastritis, n (%) | Follicular gastritis, n (%) | OR | CI | p value |
|---|---|---|---|---|---|
| CagA-/IceA– | 17 (34) | 8 (34.8) | 1 | 1 | 1 |
| CagA-/IceA+ | 4 (8) | 2 (8.7) | 1.06 | 0.16-7.06 | 0.95 |
| CagA+/IceA- | 20 (40) | 7 (30.4) | 0.74 | 0.22-2.47 | 0.63 |
| CagA+/IceA+ | 9 (18) | 6 (26.1) | 1.41 | 0.37-5.38 | 0.61 |
| VacA-s2m2 | 7 (14) | 1 (4.3) | 1 | 1 | 1 |
| VacA-s1m2 | 2 (4) | 3 (13) | 10.5 | 0.67-165.1 | 0.09 |
| VacA-s1m1 | 41 (82) | 19 (82.6) | 3.24 | 0.37-28.26 | 0.07 |
P values < 0.05 are statistically significant.
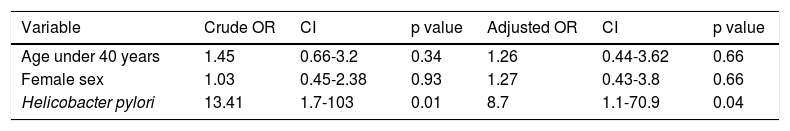
Even though the bivariate analysis showed no statistically significant associations between follicular gastritis and the age and sex variables, a review of the literature revealed a probable relation to the response variable. Therefore, the possible confounding effect of those variables was examined in a logistic regression model that included H. pylori infection (Table 6).
Multivariate model adjusted by categorized age and by sex. The measures of association of the variables of interest in relation to outcome (follicular gastritis) are shown.
| Variable | Crude OR | CI | p value | Adjusted OR | CI | p value |
|---|---|---|---|---|---|---|
| Age under 40 years | 1.45 | 0.66-3.2 | 0.34 | 1.26 | 0.44-3.62 | 0.66 |
| Female sex | 1.03 | 0.45-2.38 | 0.93 | 1.27 | 0.43-3.8 | 0.66 |
| Helicobacter pylori | 13.41 | 1.7-103 | 0.01 | 8.7 | 1.1-70.9 | 0.04 |
P values < 0.05 are statistically significant.
The relation between follicular gastritis and age has been referenced by various authors.3,8,17 Even though a young adult population would be expected in our study (given that patients with the advanced age-related conditions of atrophy, metaplasia, dysplasia, and cancer were excluded),18 the age range of the participants was from 19 to 82 years. Our results showed that, in general terms, the population with follicular gastritis was younger than the patients with chronic gastritis. That finding is similar to results published by other authors.19,20
The association between patterns of inflammatory response and ethnicity has been reported as an important risk factor for or protective factor against different diseases, including gastric cancer.21–23 For example, the risk for gastric cancer and gastric inflammation grade have recently been proposed to be a product of a phenomenon of immunologic tolerance that is dependent on the population ancestry and the bacterial ancestry of H. pylori.22 Our study participants, as well as their parents, were born in the Cauca department, and the majority of the patients with follicular gastritis were mestizo. Given the small number of patients in the present study, our conclusions are limited, but that finding is interesting, since it suggests the possibility that population ancestry and ethnic origin could influence the follicular inflammation pattern, something that has not been described in the literature.
The prevalence of Helicobacter infection in patients with follicular gastritis varies between 68% and 100%, in accordance with the number of biopsies taken.9,24 The Sydney protocol was applied in the present study, which stipulates taking 5 biopsies, enabling significant sampling of the gastric mucosa and a reliable histologic determination.25 Infection was detected through microscopic study in 97.2% of the patients with follicular gastritis, showing a significant difference with the control group (OR: 13.41, CI:1.7-103, p=0.01). That relation even persisted in the multivariate model that included the confounding variables. Likewise, a significant association was shown between inflammatory activity and the presence of bacterial infection. The link between infection and active follicular gastritis is widely recognized in the medical community and has been documented by different researchers.3,5,26,27
The role of bacterial genotypes in relation to MALT lymphoma has been evaluated in different studies. In their analyses, Eck, Ye, and Koehler, et al., were able to establish statistically significant associations between lymphoma and the cagA, vacAs1/m1, and iceA1 genotypes.28–30 However, there are few studies that relate bacterial genotypes and follicular gastritis. Specifically, Soltermann et al. evaluated the presence of lymphoid follicles in the antrum and corpus and correlated the information with the cagA and vacA alleles and genotypes, demonstrating no significant relation.31 Those findings were similar to ours. Other descriptive and analytic studies have been conducted in Latin America that show associations between bacterial genotypes and the pathologies of ulcer, cancer, and gastritis.32–35 To the best of our knowledge, the present study is the first to examine the relation of bacterial genotypes to follicular gastritis in a Colombian population.
In Colombia, Quiroga and Citelly reported cagA genotype frequencies of 50% and 51.4% in patients with gastritis,35,36 figures similar to that found in the present study (58% in the controls).
In relation to the vacAs1/m1 genotypes in the study by Citelly et al., they showed a prevalence of 43% for the s1 strain and of 40% for the m1 strain, whereas prevalence in our findings was above 80%. That information signifies that our study population had more aggressive bacterial genotypes, even at early gastric disease stages. It should be emphasized that none of the patients in the present study had atrophy, metaplasia, or dysplasia, and that the majority of patients in the case group presented with grade 2 or 3 follicular gastritis. In accordance with the knowledge there is on the cascades involved in carcinogenesis proposed by Pelayo Correa, and with the Wotherspoon model,16,37 the histologic findings in our study population would suggest a low risk for the development of cancer (both intestinal adenocarcinoma and MALT lymphoma), but the presence of cytotoxic strains would make a more careful endoscopic and histopathologic follow-up of our patients a necessity. Those genotypic differences would explain, at least partially, why patients from the Cauca department have higher incidence rates of cancer, compared with other areas of the country.15
The iceA1 genotype is considered by some authors to be a virulence factor involved in the cellular adhesion and development of ulcers, whereas others believe its prevalence depends on the geographic distribution of the bacterium.38–40 In the present study, there was no significant change in the distribution of the iceA1 genotype between the groups compared, but it was more frequent in the follicular gastritis group than in the nonatrophic gastritis group. Those data suggest a probable relation between the follicular inflammatory response and the iceA1 genotype.
The reduced number of data included for the genetic analyses was one of the limitations of our study. The verification of pH in the formalin utilized for biopsy collection or the use of transport means and culture methods as alternatives for facilitating bacterial DNA recovery are suggested for future studies. We hope to motivate the scientific community to evaluate the role of the iceA1 genotype, using larger sample sizes.
In conclusion, our study results showed that follicular gastritis and inflammatory activity are reliable markers for the presence of H. pylori infection and that the genotypes with a high oncogenic risk predominated in the study population. Therefore, its diagnosis should alert the clinician, so that progression to diseases with greater morbidity and mortality is prevented. In addition, it was suggested that the iceA genotype could play a role in the development of follicular gastritis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients and/or subjects mentioned in the article.
Financial disclosureThe present study was financed by the Programa de Salud de Colciencias, project code 1103-519-29123.
Conflict of interestThe authors of the present article declare that there is no conflict of interest.
The authors wish to thank the patients for their participation in the study and the personnel of the Hospital Universitario San José and Endovideo for their invaluable support. In addition, they wish to thank the Laboratorio de Genética Humana and the Departamento de Patología of the Facultad de Ciencias de la Salud of the Universidad del Cauca for their support in carrying out this project.
Please cite this article as: Carlosama-Rosero YH, Bolaños-Bravo H, Sierra-Tórres CH, Rosero EA. Asociación de los genotipos cagA, vacA e IceA de H. pylori con la gastritis crónica y folicular en una población colombiana con alto riesgo de cáncer gástrico. Revista de Gastroenterología de México. 2019;84:158–164.