The treatment and diagnosis of chronic diarrhea in the immunocompromised patient depends on the ability to rapidly detect the etiologic agents.
AimsOur aim was to evaluate the results of the FilmArray® gastrointestinal panel in patients newly diagnosed with HIV infection that presented with chronic diarrhea.
Material and methodsUtilizing nonprobability consecutive convenience sampling, 24 patients were included that underwent molecular testing for the simultaneous detection of 22 pathogens.
ResultsIn 24 HIV-infected patients with chronic diarrhea, enteropathogen bacteria were detected in 69% of the cases, parasites in 18%, and viruses in 13%. Enteropathogenic Escherichia coli and enteroaggregative Escherichia coli were the main bacteria identified, Giardia lamblia was found in 25%, and norovirus was the most frequent viral agent. The median number of infectious agents per patient was three (range of 0 to 7). The biologic agents not identified through the FilmArray® method were tuberculosis and fungi.
ConclusionsSeveral infectious agents were simultaneously detected through the FilmArray® gastrointestinal panel in patients with HIV infection and chronic diarrhea.
El tratamiento y el pronóstico de la diarrea crónica en el paciente inmunosuprimido dependen de la capacidad para detectar rápidamente los agentes etiológicos.
ObjetivosEvaluar los resultados del panel gastrointestinal (GI) FilmArray® en pacientes con diarrea crónica e infección por VIH de reciente diagnóstico.
Material y métodosMuestreo no probabilístico a conveniencia de manera secuencial. Se incluyeron 24 pacientes a quienes se realizó el estudio molecular para la detección simultánea de 22 patógenos.
ResultadosEn 24 pacientes infectados por el VIH con diarrea crónica se detectaron bacterias enteropatógenas en el 69% de los casos, parásitos en el 18% y virus en el 13%. Escherichia coli enteropatógena y Escherichia coli enteroagregativa fueron las principales bacterias identificadas; Norovirus fue el agente viral más frecuente encontrado (33%), seguido de Giardia lamblia (25%). La mediana del número de agentes infecciosos por paciente fue 3 (rango de 0 a 7). Los agentes biológicos no identificados por este método fueron tuberculosis y hongos.
ConclusionesUtilizando el panel GI FilmArray® se detectaron simultáneamente varios agentes infecciosos en pacientes con diarrea crónica e infección por VIH.
Diarrhea is a common morbidity and is responsible for over one million annual deaths worldwide1. If symptoms persist for more than four weeks, the condition is known as chronic diarrhea, which is a problem that affects up to 5% of the population at any given time2. It can be fatal in immunocompromised patients and occurs at a rate at least 10 times higher in individuals infected with human immunodeficiency virus (HIV), compared with the general population3. Diarrhea can affect patients with HIV at all stages of the disease and up to 60% of said patients report having symptoms of diarrhea4. Despite antiretroviral therapy (ART), HIV-associated diarrhea continues to be of multifactorial etiology that includes infectious processes, neoplasia, and enteropathy5, producing quality of life alterations, antiretroviral treatment adherence failure, weight loss, and malnutrition6,7.
The search for the etiologic agent of chronic diarrhea in the patient with HIV includes the performance of a microbiologic stool analysis (mainly stool culture, different methods for detecting intestinal parasites, and bacilloscopy), molecular methods (polymerase chain reaction [PCR]), clinical evaluation (considering the CD4 T lymphocyte count and HIV viral load determination), abdominal tomography, and colonoscopy8. Multiple infectious agents have been observed to cause chronic diarrhea and the complete clinical picture often cannot be explained by the identification of a single etiologic agent, with liquid stools persisting despite the treatment regimen, resulting in the continued performance of diagnostic tests.
Inopportune etiologic agent identification can cause significant morbidity and be responsible for approximately 80% of acquired immune deficiency syndrome (AIDS)-related deaths9. This type of diarrhea is difficult to manage and the etiologic agent is often not identified. In addition, physical examination and microbiologic test results may not be sufficient for directing therapy in some patients, at times leading the physician to resort to using antidiarrheals to improve the patient’s quality of life10.
Salmonella spp., Shigella spp., Campylobacter spp., and Mycobacterium tuberculosis are among the most frequent bacterial agents; Cystoisospora belli, Cryptosporidium parvum, Cyclospora cayetanensis, and Microsporidia are among the parasitic agents; and cytomegalovirus or herpes simplex virus type 1 and type 2 are among the viral agents. Lastly, invasive fungi, such as Histoplasma capsulatum and Paracoccidioides brasiliensis have been reported. Etiologic agent identification is generally difficult due to multiple factors, such as self-medication before seeking medical care, the poor diagnostic methods available at many laboratories, and the special analysis technique each microbial agent requires11.
One of the molecular methods available for simultaneously identifying several infectious agents is the FilmArray® Gastrointestinal (GI) Panel (MEP, BioFire Diagnostics/Biomerieux, Salt Lake City, UT, USA). It is a PCR-based technique that is useful for the rapid simultaneous detection of 22 pathogens (13 bacteria, 5 viruses, and 4 parasites) from liquid stool samples12. However, in immunocompromised patients, its true performance, compared with conventional diagnostic methods, is still unknown, especially in patients with HIV.
The aim of the present study was to evaluate the infectious agents that cause chronic diarrhea in patients with HIV infection, utilizing the FilmArray® GI Panel. Ours is the first study to utilize this molecular method for etiologic agent identification in HIV patients presenting with chronic diarrhea.
Materials and methodsStudy designAn observational, analytic, cross-sectional study was conducted on patients that were seen at the Tropical and Infectious Diseases Service of the Hospital Daniel Alcides Carrión (HDAC) in Huancayo, Peru, within the time frame of January to December 2021.The inclusion criteria were age above 18 years, HIV infection diagnosis, watery stools for more than 4 weeks, a CD4 T lymphocyte count, and viral load for HIV. Liquid stool samples were collected to identify the etiologic agent of the chronic diarrhea.
The variables of clinical data, time of disease, treatment regimen, CD4 cell count, viral load, and ART were collected. All participants gave their informed consent.
Statistical analysisNonrandomized consecutive sampling was carried out until the sample size, determined using the Stata version 14 program, was reached. To calculate the sample size, the main limiting factor was assumed to be the number of positive cases, given that its precision would depend on the identification of the infectious agent and clinical improvement after receiving the specific treatment. With a 10% margin of error, an 80% power, and a supposed minimally acceptable sensitivity of 80% for identifying the etiologic agent13,14, we calculated that a minimum of 24 patients with HIV infection and chronic diarrhea should be enrolled. The double data entry table was carried out utilizing the Excel program. The number and percentage of patients for the categorical variables and the median (interquartile range [IQR]) for the continuous variables were shown.
ProcedureAfter identifying the patients that met the inclusion criteria, they were invited to participate in the study. After providing their statements of informed consent, the patients were instructed to collect their stool in a screw cap jar for the stool culture samples. The samples were received at the laboratory and immediately processed through the FilmArray® GI Panel. Pasty or solid stool samples were rejected because the Cary-Blair medium for diluting the samples and enabling the correct processing of the panel was not available. The samples were not stored for later testing due to the fact that time and temperature could affect future procedures. Thus, all processes were carried out as soon as the samples arrived at the laboratory.
In patients that continued to present with chronic diarrhea despite receiving the treatment regimen against the infectious agents identified by the FilmArray® GI Panel, or when the panel results were negative, colonoscopy with biopsy taken at the affected area, performed by a gastroenterologist, was ordered. The modified Ziehl-Neelsen, PAS, and Grocott Gomori stains were prioritized, in addition to conventional stains, for the histopathologic study of the samples.
FilmArray® Gastrointestinal PanelThe FilmArray® GI Panel is an in vitro multiplexed diagnostic test with a closed system that contains all the reagents needed for inverse transcription, PCR, and the detection of nucleic acid of different gastrointestinal pathogens in a single stool sample. The system is approved by the FDA in the United States and by the European Union.
Said gastrointestinal panel identifies 22 pathogens that include: bacteria (Campylobacter jejuni, Campylobacter coli, Campylobacter upsaliensis, Clostridium difficile (A/B toxins), Plesiomonas shigelloides, Salmonella spp., Yersinia enterocolitica, Vibrio parahaemolyticus, Vibrio vulnificus, Vibrio cholerae, Shigella spp. and pathotypes of diarrheagenic Escherichia coli (E. coli), such as enteroaggregative E. coli (EAEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC) LT/ST, Shiga toxin-producing E. coli (STEC) stx1/stx2, E. coli O157, and enteroinvasive E. coli (EIEC); parasites (Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, Giardia lamblia), and viruses (adenovirus F 40/41, astrovirus, norovirus GI/GII, rotavirus A, sapovirus (I, II, IV, and V).
Samples were processed according to the manufacturer’s instructions (The BioFire® FilmArray® Gastrointestinal Panel). The processing time of each sample was about an hour, and the results were immediately reported to the treating physician.
A result was considered positive or negative when the final software report was obtained.
Ethical considerationsThis work was approved by the ethics committee of the Hospital Daniel Alcides Carrión (HDAC) in Huancayo, Peru, with authorization number 018-2021, respecting patient identification through codes and prior informed consent.
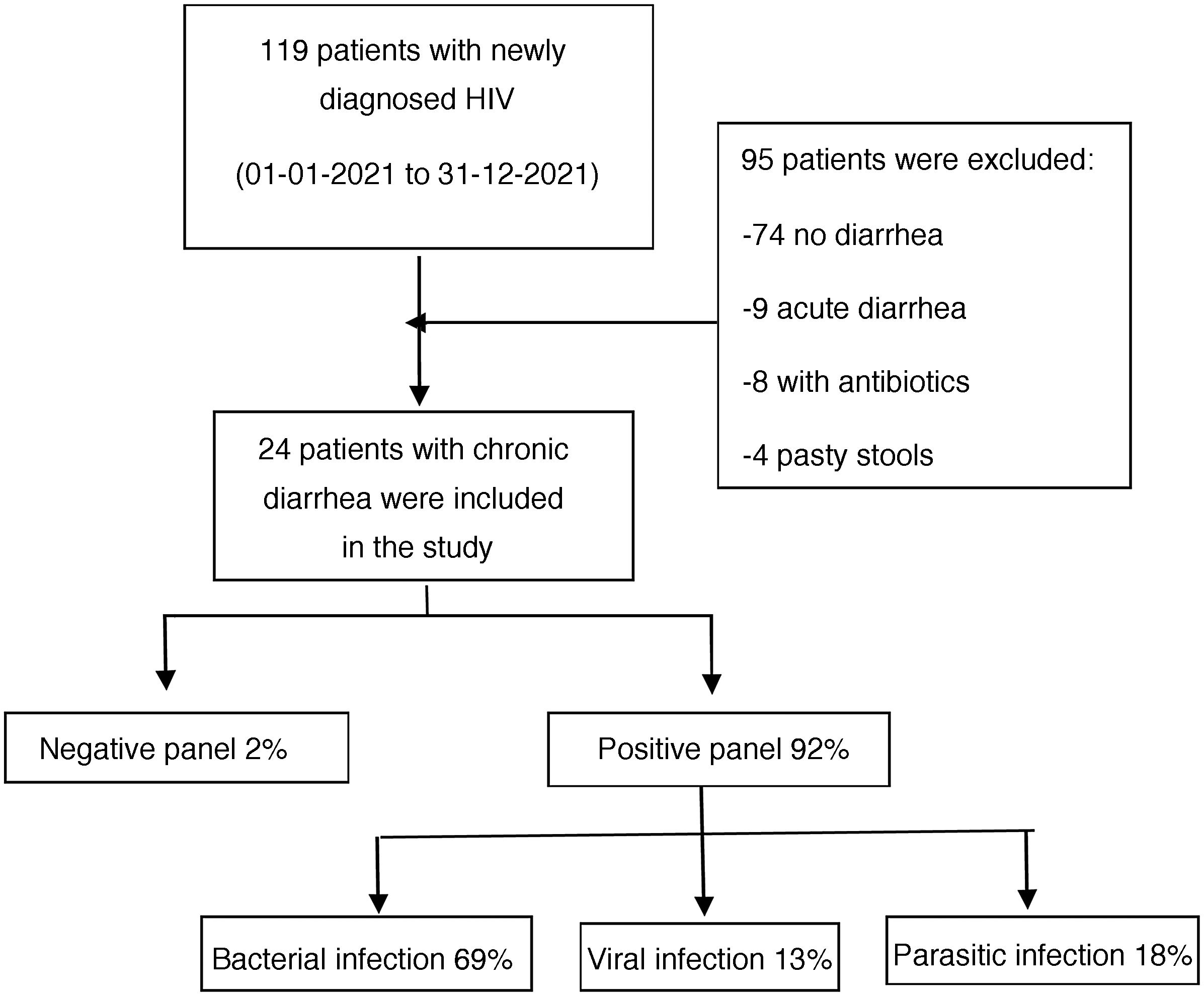
ResultsWithin the time frame of January to December 2021, 119 patients newly diagnosed with HIV were evaluated at the Infectious Diseases Service of the HDAC. Twenty-four of those patients met the inclusion criteria and were enrolled in the study (Fig. 1). All the patients fit the definition of newly diagnosed HIV infection and chronic diarrhea. Eighteen of the participants were males and the median patient age was 37 years, with an IQR of 26 to 75 years of age.
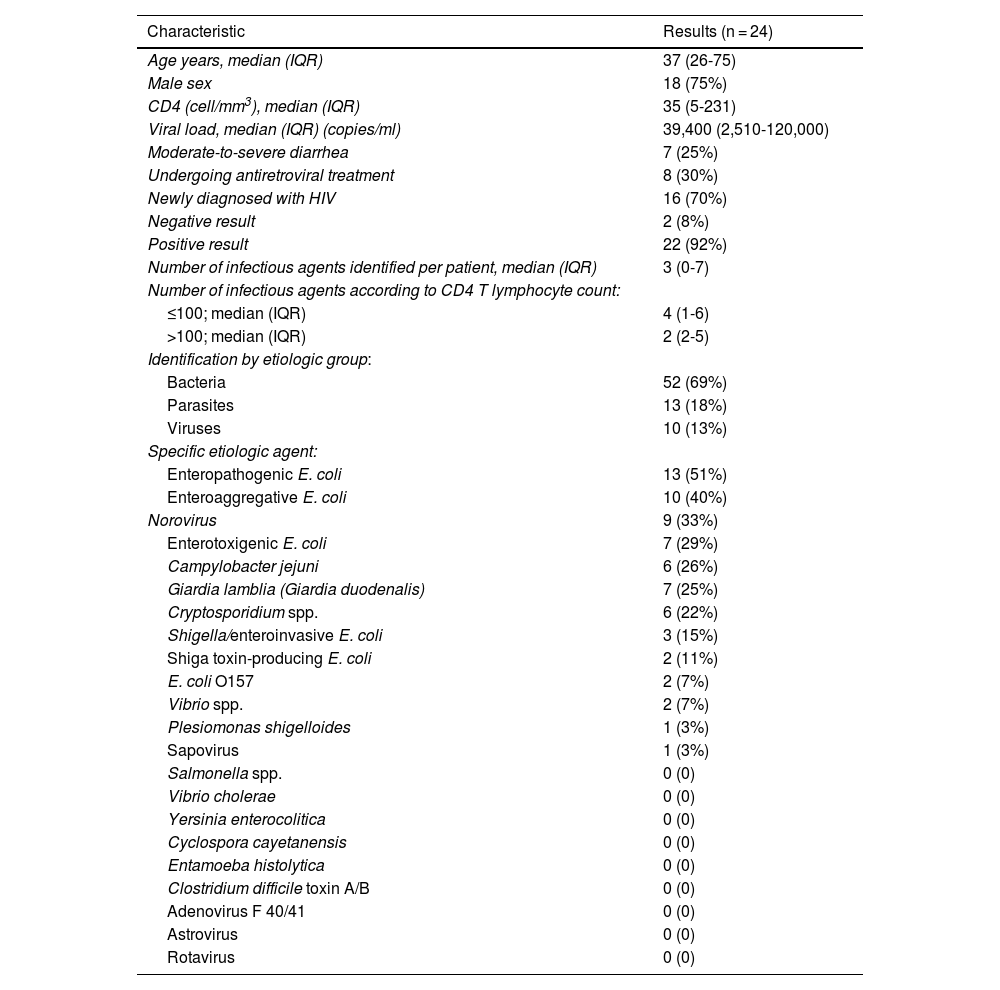
The median CD4 T lymphocyte count was 35 cells per mm3, with an IQR of 5 to 231 cells per mm3; the median viral load was 39,400 copies/ml, with an IQR of 2,510 to 120,000 copies/ml; 8 (30%) patients had started ART in the past month, all of whom took one tablet daily of the dolutegravir 50 mg/lamivudine 300 mg/tenofovir 300 mg coformulation; 16 (70%) patients were diagnosed at the Infectious Diseases Service and were not undergoing ART at the time of their enrollment in the study (Table 1).
General characteristics of the patients with HIV infection and chronic diarrhea.
| Characteristic | Results (n = 24) |
|---|---|
| Age years, median (IQR) | 37 (26-75) |
| Male sex | 18 (75%) |
| CD4 (cell/mm3), median (IQR) | 35 (5-231) |
| Viral load, median (IQR) (copies/ml) | 39,400 (2,510-120,000) |
| Moderate-to-severe diarrhea | 7 (25%) |
| Undergoing antiretroviral treatment | 8 (30%) |
| Newly diagnosed with HIV | 16 (70%) |
| Negative result | 2 (8%) |
| Positive result | 22 (92%) |
| Number of infectious agents identified per patient, median (IQR) | 3 (0-7) |
| Number of infectious agents according to CD4 T lymphocyte count: | |
| ≤100; median (IQR) | 4 (1-6) |
| >100; median (IQR) | 2 (2-5) |
| Identification by etiologic group: | |
| Bacteria | 52 (69%) |
| Parasites | 13 (18%) |
| Viruses | 10 (13%) |
| Specific etiologic agent: | |
| Enteropathogenic E. coli | 13 (51%) |
| Enteroaggregative E. coli | 10 (40%) |
| Norovirus | 9 (33%) |
| Enterotoxigenic E. coli | 7 (29%) |
| Campylobacter jejuni | 6 (26%) |
| Giardia lamblia (Giardia duodenalis) | 7 (25%) |
| Cryptosporidium spp. | 6 (22%) |
| Shigella/enteroinvasive E. coli | 3 (15%) |
| Shiga toxin-producing E. coli | 2 (11%) |
| E. coli O157 | 2 (7%) |
| Vibrio spp. | 2 (7%) |
| Plesiomonas shigelloides | 1 (3%) |
| Sapovirus | 1 (3%) |
| Salmonella spp. | 0 (0) |
| Vibrio cholerae | 0 (0) |
| Yersinia enterocolitica | 0 (0) |
| Cyclospora cayetanensis | 0 (0) |
| Entamoeba histolytica | 0 (0) |
| Clostridium difficile toxin A/B | 0 (0) |
| Adenovirus F 40/41 | 0 (0) |
| Astrovirus | 0 (0) |
| Rotavirus | 0 (0) |
IQR: interquartile range.
According to the etiologic agent grouped by type of infectious microorganism, in the positive samples, bacteria were responsible for the chronic diarrhea in 69% of the patients, parasites were identified in 18%, and viruses in 13%.
In the 24 watery stool samples that were positive, E. coli was the most frequently identified bacterial agent in chronic diarrhea and the most frequent of those diarrheagenic pathotypes were EPEC, EAEC, and ETEC, at values of 51%, 40%, and 29%, respectively. The second most frequently identified bacterium was Campylobacter jejuni, found in 26% of the samples. Norovirus was the most prevalent of the viral agents, detected in 33% of the samples, and sapovirus was identified in 3%.
Giardia lamblia was the most frequent parasite, found in 25% of the samples, followed by Cryptosporidium spp., identified in 22% of the patients with chronic diarrhea.
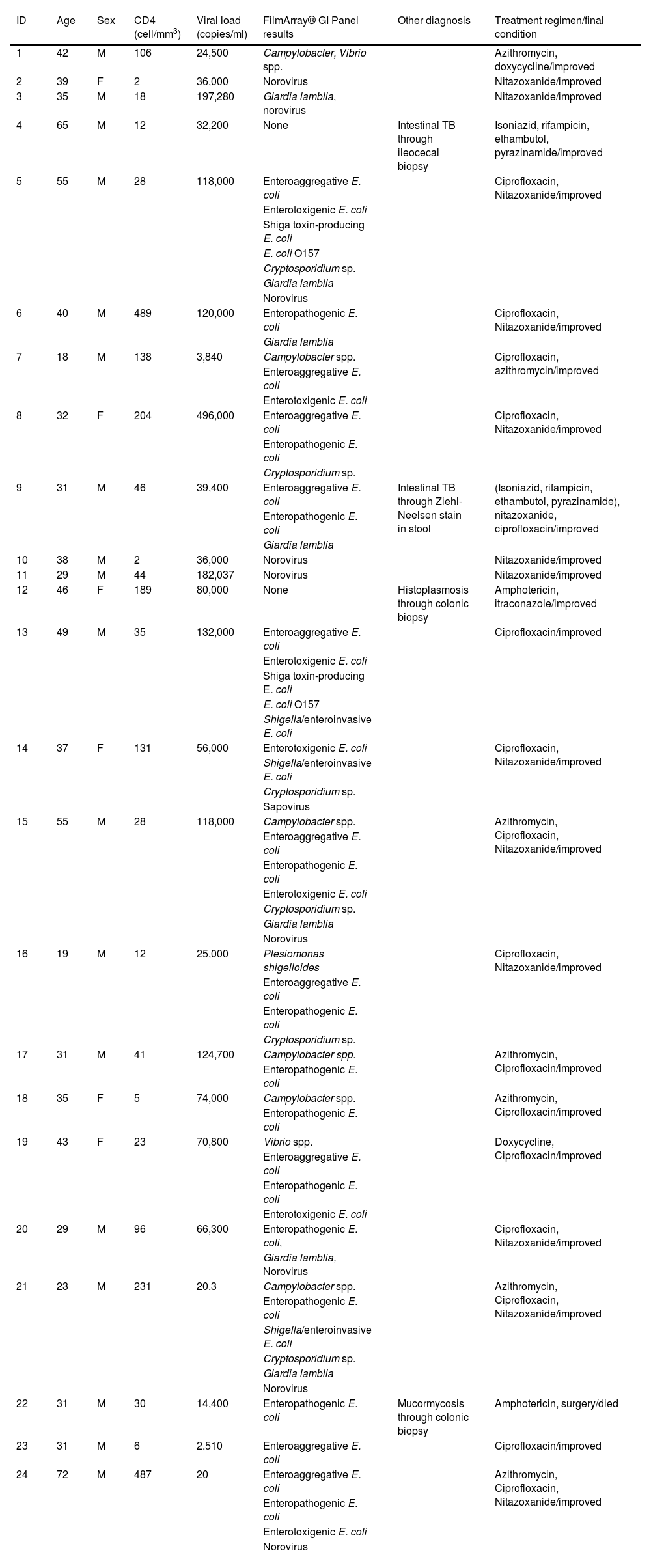
Three was the median number of infectious agents found in a single patient with HIV, with a range of 0 to 7 infectious microorganisms per patient. In the 24 patients analyzed, 2 (8%) had negative samples and 22 (92%) had positive samples. Table 2 provides a detailed summary of the performance of the FilmArray® GI Panel for each of the 24 patients.
Etiologic agent identification results using the FilmArray® GI Panel in patients with HIV infection and chronic diarrhea.
| ID | Age | Sex | CD4 (cell/mm3) | Viral load (copies/ml) | FilmArray® GI Panel results | Other diagnosis | Treatment regimen/final condition |
|---|---|---|---|---|---|---|---|
| 1 | 42 | M | 106 | 24,500 | Campylobacter, Vibrio spp. | Azithromycin, doxycycline/improved | |
| 2 | 39 | F | 2 | 36,000 | Norovirus | Nitazoxanide/improved | |
| 3 | 35 | M | 18 | 197,280 | Giardia lamblia, norovirus | Nitazoxanide/improved | |
| 4 | 65 | M | 12 | 32,200 | None | Intestinal TB through ileocecal biopsy | Isoniazid, rifampicin, ethambutol, pyrazinamide/improved |
| 5 | 55 | M | 28 | 118,000 | Enteroaggregative E. coli | Ciprofloxacin, Nitazoxanide/improved | |
| Enterotoxigenic E. coli | |||||||
| Shiga toxin-producing E. coli | |||||||
| E. coli O157 | |||||||
| Cryptosporidium sp. | |||||||
| Giardia lamblia | |||||||
| Norovirus | |||||||
| 6 | 40 | M | 489 | 120,000 | Enteropathogenic E. coli | Ciprofloxacin, Nitazoxanide/improved | |
| Giardia lamblia | |||||||
| 7 | 18 | M | 138 | 3,840 | Campylobacter spp. | Ciprofloxacin, azithromycin/improved | |
| Enteroaggregative E. coli | |||||||
| Enterotoxigenic E. coli | |||||||
| 8 | 32 | F | 204 | 496,000 | Enteroaggregative E. coli | Ciprofloxacin, Nitazoxanide/improved | |
| Enteropathogenic E. coli | |||||||
| Cryptosporidium sp. | |||||||
| 9 | 31 | M | 46 | 39,400 | Enteroaggregative E. coli | Intestinal TB through Ziehl-Neelsen stain in stool | (Isoniazid, rifampicin, ethambutol, pyrazinamide), nitazoxanide, ciprofloxacin/improved |
| Enteropathogenic E. coli | |||||||
| Giardia lamblia | |||||||
| 10 | 38 | M | 2 | 36,000 | Norovirus | Nitazoxanide/improved | |
| 11 | 29 | M | 44 | 182,037 | Norovirus | Nitazoxanide/improved | |
| 12 | 46 | F | 189 | 80,000 | None | Histoplasmosis through colonic biopsy | Amphotericin, itraconazole/improved |
| 13 | 49 | M | 35 | 132,000 | Enteroaggregative E. coli | Ciprofloxacin/improved | |
| Enterotoxigenic E. coli | |||||||
| Shiga toxin-producing E. coli | |||||||
| E. coli O157 | |||||||
| Shigella/enteroinvasive E. coli | |||||||
| 14 | 37 | F | 131 | 56,000 | Enterotoxigenic E. coli | Ciprofloxacin, Nitazoxanide/improved | |
| Shigella/enteroinvasive E. coli | |||||||
| Cryptosporidium sp. | |||||||
| Sapovirus | |||||||
| 15 | 55 | M | 28 | 118,000 | Campylobacter spp. | Azithromycin, Ciprofloxacin, Nitazoxanide/improved | |
| Enteroaggregative E. coli | |||||||
| Enteropathogenic E. coli | |||||||
| Enterotoxigenic E. coli | |||||||
| Cryptosporidium sp. | |||||||
| Giardia lamblia | |||||||
| Norovirus | |||||||
| 16 | 19 | M | 12 | 25,000 | Plesiomonas shigelloides | Ciprofloxacin, Nitazoxanide/improved | |
| Enteroaggregative E. coli | |||||||
| Enteropathogenic E. coli | |||||||
| Cryptosporidium sp. | |||||||
| 17 | 31 | M | 41 | 124,700 | Campylobacter spp. | Azithromycin, Ciprofloxacin/improved | |
| Enteropathogenic E. coli | |||||||
| 18 | 35 | F | 5 | 74,000 | Campylobacter spp. | Azithromycin, Ciprofloxacin/improved | |
| Enteropathogenic E. coli | |||||||
| 19 | 43 | F | 23 | 70,800 | Vibrio spp. | Doxycycline, Ciprofloxacin/improved | |
| Enteroaggregative E. coli | |||||||
| Enteropathogenic E. coli | |||||||
| Enterotoxigenic E. coli | |||||||
| 20 | 29 | M | 96 | 66,300 | Enteropathogenic E. coli, | Ciprofloxacin, Nitazoxanide/improved | |
| Giardia lamblia, Norovirus | |||||||
| 21 | 23 | M | 231 | 20.3 | Campylobacter spp. | Azithromycin, Ciprofloxacin, Nitazoxanide/improved | |
| Enteropathogenic E. coli | |||||||
| Shigella/enteroinvasive E. coli | |||||||
| Cryptosporidium sp. | |||||||
| Giardia lamblia | |||||||
| Norovirus | |||||||
| 22 | 31 | M | 30 | 14,400 | Enteropathogenic E. coli | Mucormycosis through colonic biopsy | Amphotericin, surgery/died |
| 23 | 31 | M | 6 | 2,510 | Enteroaggregative E. coli | Ciprofloxacin/improved | |
| 24 | 72 | M | 487 | 20 | Enteroaggregative E. coli | Azithromycin, Ciprofloxacin, Nitazoxanide/improved | |
| Enteropathogenic E. coli | |||||||
| Enterotoxigenic E. coli | |||||||
| Norovirus |
CD4: CD4 T lymphocyte count; F: female; M: male.
Two of the 24 patients did not respond to the treatment regimen against the identified infectious agent and a pathogen could not be identified in another two patients, and so those four patients underwent colonoscopy. M. tuberculosis was found in two of them, Histoplasma spp. in one, and mucormycosis in one (Table 2).
DiscussionConcurrent with previous studies, diarrhea is the most frequent morbidity in patients with HIV infection, and without ART, there is up to an 80% possibility of developing chronic diarrhea. The most frequent causes of such enteric infections are mainly bacterial in origin15. In one study, parasites were the main cause of chronic diarrhea16, whereas in another, tuberculosis was the primary cause17, each with a different epidemiologic history.
Despite all the existing methods, there is still a group of patients in whom the pathogenic agent that causes chronic diarrhea cannot be identified, resulting in malnutrition and physical deterioration, known as HIV enteropathy18, and becoming a public health problem because of the inadequate use of numerous antibiotics and increased antimicrobial resistance. Starting ART early is known to decrease those infections, but it is also related to the ability to detect the etiologic agents associated with chronic diarrhea.
The FilmArray® GI Panel has been evaluated for detecting the infectious agent in enteritis in immunocompromised patients and has 100% sensitivity and 97% specificity, when compared with conventional cultures19. It also has a capacity of up to 40% for detecting agents that are not commonly studied20.
However, there are few reports on immunocompromised patients; in one case of multiple myeloma with chronic diarrhea, the agent identified using the FilmArray® GI Panel was E. coli21. In our study, the agents most frequently identified were bacteria, similar to that reported in a study from the United States in patients with chronic diarrhea, which through stool cultures, identified Salmonella spp. (mainly Salmonella enterica, Salmonella typhimurium, and Salmonella enteritidis) in first place. However, the isolation of E. coli, particularly EAEC, showed it was the pathogen that could contribute to a greater development of diarrheal disease, by reducing local immunity and prolonging disease chronicity22. Nevertheless, culture use is still poorly understood, given that it continues to be a test with a questionable yield.
The high level of E. coli as a cause of enteric infections in our group of patients may be due to the ingestion of contaminated foods or water23, severe immunosuppression, and the practice of unsafe anal sex resulting in fecal-oral exposure. Unsafe anal sex is related to infection with Campylobacter jejuni24, which was the second most frequent type of bacteria identified by the FilmArray® GI Panel.
Said panel detected multiple types of pathogens in one test. In addition to the enteropathogenic bacteria, E. coli or Campylobacter spp., it identified Giardia lamblia as the most frequent parasite, followed by Cryptosporidium spp. Our findings differed from those of an Asian study that found Cryptosporidium spp. as the main pathogen related to chronic diarrhea in HIV patients, surpassing enteropathogenic bacteria and viruses25.
Norovirus was the most frequent viral agent, followed by sapovirus. In a previous Chinese study that utilized the FilmArray® GI Panel, norovirus was identified as the main infectious agent in 53% of the study population in patients with HIV and diarrhea, but the etiologic agents were not differentiated according to acute and chronic diarrhea types26. Norovirus can produce acute disease, but its chronicity might be due to the GII genotype and coinfection with other viral agents, mainly rotavirus, sapovirus, astrovirus, and adenovirus27.
The presence of multiple coexisting pathogens can also create a climate of symbiosis, favoring its permanence, causing effects on the cell transporters that are similar to those caused by the toxins, prolong the destructive and inflammatory effect on the intestinal villi, and produce binding proteins that facilitate adherence of other pathogens in the apical membranes of enterocytes28,29.
In our study, two patients had a negative FilmArray® GI Panel result, signifying that it did not detect the entire spectrum of pathogens related to chronic diarrhea in patients with HIV, making it necessary to continue investigating the presence of other pathogens through colonoscopy, mainly in cases of persistent diarrhea.
The limitation of the present study is the fact that the FilmArray® GI Panel does not detect certain opportunistic pathogens in patients with HIV, such as microsporidia, Cystoisospora belli, tuberculosis, and histoplasma. Therefore, conventional studies should be performed in parallel with the FilmArray® GI Panel, such as parasite exams with special dyes for detecting tuberculosis and Cystoisospora belli (modified Ziehl-Neelsen stain), and patients that continue to present with diarrhea, despite undergoing the treatment regimen, should undergo biopsy of the affected colonic mucosa through colonoscopy. In our study, colonoscopy performed on the patients that did not respond to treatment helped to identify other causes of chronic diarrhea, such as tuberculosis, histoplasma, and mucormycosis.
ConclusionOur study results suggest that by utilizing the FilmArray® GI Panel, multiple pathogens associated with chronic diarrhea in patients with HIV can be identified in a single test, facilitating diagnosis and early treatment, and its usefulness in HIV patients justifies its cost. Conventional studies, such as Ziehl-Neelsen staining in stools, should also be maintained. In addition, if chronic diarrhea persists, we recommend studying the patient through colonoscopy for other nonidentified agents, such as tuberculosis, histoplasma, and cytomegalovirus.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.