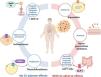
Diabetes is a public health problem with an estimated worldwide prevalence of 10% and a prevalence of 12% in Mexico. The costs resulting from this chronic-degenerative disease are significant. Treatment for diabetes involves different medication groups, some of which can cause significant gastrointestinal adverse effects, such as dyspepsia, nausea, vomiting, bloating, diarrhea, and constipation. The medications most frequently associated with said adverse effects are metformin, acarbose, and GLP-1 agonists. Gastrointestinal adverse effects negatively impact the quality of life and management of patients with diabetes. The factors of visceral neuropathy, acute dysglycemia, dysbiosis, and intestinal bacterial overgrowth contribute to the gastrointestinal symptoms in patients with diabetes, making it necessary to consider multiple etiologic factors in the presence of gastrointestinal symptoms, and not exclusively attribute them to the use of antidiabetics. Personalized treatment, considering gastrointestinal comorbidity and the type of drug utilized, is essential for mitigating the adverse effects and improving the quality of life in patients with diabetes. The aim of the present narrative review was to describe the gastrointestinal adverse effects of the antidiabetic drugs, their pathophysiologic mechanisms, and the corresponding therapeutic measures.
La diabetes es un problema de salud pública con prevalencias globales estimadas del 10% y en México del 12%. Esta enfermedad crónico-degenerativa genera costos significativos. El tratamiento para la diabetes involucra diversos grupos de medicamentos, algunos de los cuales pueden provocar efectos adversos gastrointestinales significativos, como dispepsia, náuseas, vómitos, distensión abdominal, diarrea y estreñimiento. Los medicamentos asociados con mayor frecuencia a dichos efectos adversos son la metformina, acarbosa y agonistas GLP-1. Los efectos adversos gastrointestinales impactan en la calidad de vida y en el manejo de los pacientes con diabetes. Factores como la neuropatía visceral, disglucemia aguda, disbiosis y el sobrecrecimiento bacteriano intestinal también contribuyen a los síntomas gastrointestinales en pacientes con diabetes por lo que se deben de considerar múltiples opciones etiológicas ante síntomas gastrointestinales y no atribuirlos exclusivamente al uso de antidiabéticos. La individualización del tratamiento, considerando la comorbilidad gastrointestinal y el tipo de fármaco utilizado, es crucial para mitigar los efectos adversos y mejorar la calidad de vida de los pacientes con diabetes. Esta revisión narrativa tiene como objetivo describir los efectos adversos gastrointestinales de los antidiabéticos, así como sus mecanismos fisiopatológicos y las medidas terapéuticas correspondientes.
Diabetes is a national and worldwide public health problem, with a reported global prevalence of 10.5% and a prevalence of 12.6% in Mexico.1–3 The high prevalence of this chronic-degenerative disease results in important healthcare costs in Mexico and accounts for 14% of deaths. The mean accumulated costs produced by type 2 diabetes per patient at 10 and 20 years is 2,302.51 USD and 4,398.7 USD, respectively.4 There are currently 12 medication groups approved for the management of diabetes and some of them have been associated with significant gastrointestinal adverse effects; the most frequently involved are GLP-1 agonists, metformin, and alpha-glucosidase inhibitors. Symptoms of dyspepsia, nausea, vomiting, bloating, diarrhea, and constipation stand out among the gastrointestinal adverse effects described.5–14
The presence of gastrointestinal adverse effects associated with antidiabetics has an overall impact on the patient with diabetes, given that they can increase the need for medical attention, affect quality of life due to the symptoms they trigger, and modify therapeutic regimens. Hence our interest in carrying out a narrative review on this theme, in which we aimed to describe the gastrointestinal adverse effects of antidiabetics, their pathophysiologic mechanisms, and the therapeutic measures to be carried out.
MethodologyAn information synthesis on the theme “Gastrointestinal adverse effects of antidiabetics” was developed through a narrative review. An online search utilizing the PubMed® search engine was carried out using the MeSH terms “Diabetes Mellitus”, “Hyperglycemia”, “Diabetes Complications”, “Hypoglycemic Agents”, “Glucagon-Like Peptide-1 Receptor Agonists”, “Glycoside Hydrolase Inhibitors”, “Acarbose”, “Metformin”, “Dipeptidyl-Peptidase IV Inhibitors”, “Sodium-Glucose Transporter 2 Inhibitors”, “Drug-Related Side Effects and Adverse Reactions”, “Gastrointestinal Diseases”, “Gastroparesis”, “Gastric Emptying”, “Dysbiosis”, “Gastrointestinal Microbiome”, “Microbiota”, “Constipation”, “Diarrhea”, “Nausea”, “Vomiting”, “Gastrointestinal Agents”. Experimental articles, clinical trials, systematic reviews, meta-analyses, clinical practice guidelines, consensuses, and three narrative reviews for terminology references were included.
Within the time frame of January 2023 and January 2024, three sessions were held that included four gastroenterology specialists and three endocrinology specialists, for the purpose of reviewing and discussing the scientific evidence collected.
Factors associated with the appearance of gastrointestinal symptoms in patients with diabetesEven though gastrointestinal symptoms are traditionally associated with patients living with diabetes, the reported prevalence in different population groups varies.15 Distinct factors, in addition to the drugs themselves for managing the disease, can cause gastrointestinal manifestations, making the approach to the patients with diabetes and gastrointestinal symptoms a challenge. The neuronal dysfunction that characterizes diabetes,16 hyperglycemia, and dietary modifications that include the use of sweeteners, as well as a greater predisposition to certain psychologic disorders,16 are some of the factors that can independently contribute to or interact together for the development and progression of gastrointestinal symptoms in this group of patients with psychologic disorders.16,17
Diabetic gastrointestinal neuropathyDiabetic gastrointestinal neuropathy can affect any part of the gastrointestinal tract, manifesting as gastroparesis, constipation, or fecal incontinence secondary to visceral neuropathy.16,18 Chronic hyperglycemia conditions an altered enteric microenvironment, with increased oxidative stress, the formation of advanced glycosylation end products, inflammation, and reduced neurotransmitters and local hormones, affecting the enteric vasculature and smooth muscle cellularity.19 Previously described changes affect the sensory, motor, and secretory functions of the digestive tract, contributing to the wide range of gastrointestinal alterations mentioned.18
Gastroparesis is one of the most widely studied gastrointestinal manifestations of diabetic gastrointestinal neuropathy. Classically, diabetic gastroparesis has been described as the slowing down of gastric motility in patients with diabetes, mainly associated with poorly controlled chronic hyperglycemia.20 Gastroparesis is defined as an abnormal delay in gastric emptying, in the absence of mechanical obstruction.21 Of the 30 to 50% of patients with longstanding diabetes, gastric emptying delay may or may not be associated with the gastrointestinal symptoms of nausea, vomiting, bloating, early satiety, and postprandial fullness.22,23 However, the presence of diabetic gastroparesis varies, according to the population analyzed and the diagnostic methods employed in each study.24
Acute dysglycemiaAcute dysglycemia, i.e., acute episodes of either hyperglycemia or hypoglycemia, affect gastrointestinal motor function and intestinal sensitivity.25 Acute hyperglycemia has been associated with delayed gastric emptying or ileus.26 During episodes of diabetic ketoacidosis, 46% of patients present with abdominal pain, which, in large part, can be explained by gastric emptying delay and the electrolytic abnormalities the episodes tend to present with, such as hypokalemia. In contrast, acute hypoglycemia has been associated with increased gastric emptying.27,28 Changes in gastric emptying associated with acute dysglycemia have been proposed as an additional form of glucose regulation, in which glucose absorption is increased or reduced, as a counterregulatory response, according to glucose requirements.24
Dysbiosis and bacterial overgrowthAnother of the manifestations associated with diabetic gastrointestinal neuropathy is the slowing down of intestinal transit, propitiating the intestinal bacterial overgrowth that favors intestinal malabsorption and chronic diarrhea.15 In addition to neuropathy, there are other factors that have been associated with bacterial overgrowth in patients with diabetes, among which are reduced pancreatic exocrine function, as well as chronic opioid use.29,30
Use of artificial sweetenersDespite the fact that artificial sweeteners as an alternative to sugar appeared to be an adequate strategy for glycemic control and reduced calorie intake, in recent years, their use has been linked to undesirable metabolic effects, including gastrointestinal effects.31 Intestinal motility alterations and changes in the gut microbiota have been more frequently reported through experimental models but results in clinical trial results have not been conclusive.32
Psychologic dysfunctionThe psychologic disorders of anxiety and depression have been reported to be highly prevalent in patients with diabetes.17 In turn, those same psychologic comorbidities are strongly associated with gastrointestinal symptoms, increasing their appearance and perception.33 Elevated levels of anxiety, depression, and neurosis have previously been described to be directly related to gastrointestinal symptoms in patients with diabetes, suggesting an additional nonorganic factor of gastrointestinal dysfunction associated with diabetes.34,35
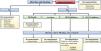
Antidiabetic pharmacologic generalitiesIt is important to remember the pharmacologic classification of antidiabetics and recognize that some of them have gastrointestinal effects, such as the biguanides, alpha-glucosidase inhibitors, and GLP-1 analogues (Fig. 1).
MetforminMetformin is a biguanide with a complex mechanism of action that produces multiple biologic effects, but the main mechanism of action that has been described is through the adenosine monophosphate-activated protein kinase (AMPK) enzymatic complex. The AMPK complex regulates the activity of numerous transcription factors involved in lipid metabolism, inflammation, autophagy, and gluconeogenesis.36 At the level of the liver, gluconeogenesis is reduced due to mitochondrial complex 1 inhibition, generating an increase in potential cell reduction (NADH: NAD), as well as in mitochondrial complex IV due to the inhibition dependent on glycerol-3-phosphate dehydrogenase (mGPHD ).37 In brown fatty tissue, this biguanide reduces the proinflammatory status conditioned by M1 macrophages through hypoxia-inducible factor 1 alpha (HIF-1 alpha), restoring the beta-adrenergic response.38 In the digestive tract, it induces a transitory inhibition of glucose absorption and abundance of the sodium-glucose transporter 1 (SGLT1) in the apical membrane of the jejunal enterocytes.37
AcarboseAcarbose is an alpha-glucosidase inhibitor, a group of medications that makes up part of the oral antidiabetics that act through the competitive and reversible inhibition of intestinal alpha-glucosidase.39 Alpha-glucosidase is an intestinal enzyme that favors glucose absorption through the enzymatic degradation of polysaccharides and disaccharides into said monosaccharide. Even though it is a drug with few systemic adverse effects, the gastrointestinal symptoms that are triggered have been considered a recurrent problem with this group of medications.6,7
Dipeptidyl peptidase 4 inhibitorsIncretins are intestinal peptides that are secreted after eating foods that, together with hyperglycemia, stimulate insulin release and play an essential role in glucose homeostasis. These peptides, which include glucose-dependent insulinotropic peptide (GIP) and GLP1, are responsible for the incretin effect, which explains why the stimulus for insulin release is stronger upon receiving an oral glucose load, rather than an intravenous one.40,41
Dipeptidyl peptidase 4 (DPP-4) is the protein that cleaves and inactivates GLP-1 and GIP in a few minutes,40 thus the medications that inhibit said aminopeptidase increase the circulating levels of the incretins, enabling the stimulation of glucose-dependent insulin secretion in the pancreatic islets, through membrane receptors in the β cells, to persist.41
Sitagliptin, linagliptin, saxagliptin, vildagliptin, and alogliptin, among others, belong to the group of DPP-4 inhibitors (iDDP-4).
Unlike other medications utilized in the management of diabetes, but like the information on SGLT2 inhibitors, there is little evidence on the association of that pharmacologic group with gastrointestinal symptomatology.42
Sodium-glucose cotransporter type 2 inhibitorsSodium-glucose cotransporter type 1 (SGLT1) and type 2 (SGLT2) are members of the SLC5 gene family, a subdivision of a superfamily of sodium cotransporters. Type 1 is expressed in the brush border membrane of the small bowel enterocytes and to a larger degree, in the renal cortex, specifically in the S3 segment of the luminal membrane. On the other hand, the SGLT2 is exclusively confined to the luminal membrane of the S1 and S2 segments of the renal proximal tubule. The first four medications inhibiting SGLT2 to be approved were dapagliflozin, canagliflozin, empagliflozin, and sotagliflozin. There is evidence on changes in the gut microbiota of patients being treated with SGLT2 inhibitors, but no evidence that supports an association with gastrointestinal adverse effects.5,43,44
GLP-1 receptor analoguesThe glycogen-like peptide type 1 (GLP-1) receptor analogues are a group of medications that are useful for treating diabetes and their administration route is oral or subcutaneous. Numerous effects are responsible for the mechanism of action of these drugs. They include the incretin effect, with the enhancement of insulin secretion by beta cells, improved insulin sensitivity, decrease in glucagon secretion, and reduced food intake due to induced satiety.11,45 The effect of this drug group on weight loss has had a worldwide impact due to its misuse through self-medication, and in turn, an increase in the number of cases of patients with adverse effects.12,46–48
Approach to gastrointestinal symptoms in patients that are candidates for or receivers of antidiabetic treatmentWhen evaluating patients with gastrointestinal symptoms that are going to start medical management for diabetes, it is important to ask if there are symptoms or diagnoses of functional dyspepsia, gastroparesis, intestinal bacterial overgrowth, irritable bowel syndrome, bloating, or chronic constipation, given that a large part of the therapeutic options can trigger or exacerbate symptomatology, such as early satiety, postprandial fullness, nausea, vomiting, bloating, diarrhea, and constipation.49–51
In the case of patients that seek medical evaluation due to symptomatology that began after the start of pharmacologic management, the initial approach should focus on the symptoms and not associate them with the drugs as a first possibility, directly looking for alarm signs that merit endoscopy or colonoscopy.49
It is important to consider the drug group being utilized because different drugs among the different groups are associated with more symptoms than others, signifying that the molecule used can be modified, before suspending the drug group.5,42 Likewise, the initial dose and adequate drug titration should be evaluated, given that in some cases, adequate dose escalation of the drug can reduce or prevent gastrointestinal adverse effects, as occurs with the GLP-1 agonists.52
The temporality of the appearance of symptoms should be evaluated because in some cases the symptomatology tends to present in the first days of application. In such cases, applying prophylactic regimens for short periods of time can be beneficial.48
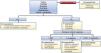
Therapeutic measures are individualized, depending on the gastrointestinal comorbidities, current diet, predominant symptom, and drug group utilized (Figs. 2 and 3).
Approach to gastrointestinal symptoms in patients eligible for or undergoing treatment with GLP-1 agonists.
UGIS: upper gastrointestinal symptoms.
*Family history of colon or stomach cancer, > 50 years of age, iron deficiency anemia, nocturnal symptoms, weight loss, gastrointestinal bleeding, persistent vomiting, palpable epigastric mass, or dysphagia.
¿Nausea, vomiting, dyspepsia, heartburn, regurgitation.
The cause of the adverse effects related to the drugs utilized in the management of diabetes is multifactorial, including genetic predisposition, glycemic control, diabetic gastroparesis diagnosis, and dose and pharmacokinetics of the drug employed.
MetforminBecause extended-release metformin, compared with the immediate-release presentation, is associated with a lower incidence of gastrointestinal adverse effects, such as nausea, vomiting, dyspepsia, and diarrhea, opting for the extended-release presentation is the first step in preventing the adverse effects related to this medication.9,10
The mechanism of action through which metformin triggers gastrointestinal adverse effects is not known, but there is increasing evidence supporting the idea that the pathophysiologic mechanism is secondary to dysbiosis, given the reports that Intestinibacter spp. and Clostridium spp. are reduced and Escherichia/Shigella spp. and Bilophila wadsworthia are increased.53–56 Thus, the combination of metformin with prebiotics and probiotics has been proposed as a possible way to reduce the risk of gastrointestinal adverse effects. A randomized controlled trial conducted by Dixon et al.57 showed that the dual administration of prebiotics and metformin produced favorable changes in the microbiota but did not reduce the gastrointestinal adverse effects.
Regarding the use of probiotics, in a randomized controlled trial carried out by Şahin et al.58 the concomitant use of metformin and Bifidobacterium animalis subsp. lactis (BB-12), compared with metformin monotherapy, was shown to be associated with fewer alterations involving the sense of taste, abdominal pain, and subjective bloating. Likewise, in a meta-analysis conducted by Memon et al.59 that included 17 clinical trials and a total of 1,009 patients, the concomitant use of metformin with probiotics was associated with fewer gastrointestinal adverse effects (OR 0.18, 95% CI 0.09-0.3.8).
AcarboseEven though acarbose has been shown to produce changes in the composition of the microbiota, dysbiosis and intestinal bacterial overgrowth are not the pathophysiologic mechanisms involved in the bloating and diarrhea associated with the administration of the drug.6,7,60–64 The acarbose-associated alteration of the microbiota is an increase in the density of the genus Lactobacillus and the genus Bifidobacterium, a composition of the microbiota that is protective for gastrointestinal symptomatology.64,65 Despite the protective modification in the microbiota, acarbose is associated with gastrointestinal adverse effects by producing poor disaccharide, oligosaccharide, and polysaccharide absorption. Although there is no evidence on a low FODMAP diet or one that is selective of its components, there is theoretically supported evidence on utilizing that intervention as a therapeutic measure for treating or preventing abdominal pain, bloating, and diarrhea in that group of patients.66–70
DPP4 inhibitorsUnlike other drugs utilized in the management of diabetes, the DPP4 inhibitors are not associated with gastrointestinal adverse effects, or their association is minimal. The gastrointestinal adverse effects are attributed to the GLP-1 activity these medications produce and there are no therapeutic measures for improving said symptomatology.42,71–73 Therefore, the best measure to consider in this group of patients is to use the drug that has less association with gastrointestinal adverse effects. In order of frequency, the DPP4 inhibitors with less symptomatic association are vildagliptin, alogliptin, sitagliptin, saxagliptin, linagliptin, and teneligliptin.42
SGLT2 inhibitorsAs with the DPP4 inhibitors, the SGLT2 inhibitors have not been associated with gastrointestinal adverse effects, or their association is minimal. However, they have been shown to induce a compositional change in the gut microbiota.5,43,44,74 Said changes in the gut microbiota are the increase in short-chain fatty acid-producing bacteria, such as Eubacterium, Roseburia, and Faecalibacterium, which in turn, is associated with bloating, diarrhea, or constipation, due to modifications in intestinal transit.43,75,76 There is little evidence for implementing therapeutic or prophylactic measures in the management of gastrointestinal symptomatology associated with the SGLT2 inhibitors, but there are theoretical bases that a low-FODMAP diet could be useful in those patients.75–77
GLP-1 analoguesThe first step for preventing adverse effects related to the GLP-1 antagonists is to avoid their use in patients with suspected gastroparesis, postprandial distress syndrome (a subtype of functional dyspepsia), or chronic constipation, to not exacerbate those disorders.46,78
Among the first considerations in the therapeutic approach to GLP-1 agonist-related nausea and vomiting, is evaluating the glycemic control of the patient, given that glucose levels > 288 mg/dl are associated with delayed gastric emptying; such patients can present with postprandial fullness, nausea, or vomiting due to a lack of glycemic control, rather than an adverse effect of the medication.79–82
One of the pathophysiologic mechanisms of those adverse effects is the delay in gastric emptying, and so it is important to reinforce hygienic-postural and pharmacologic measures for gastroesophageal reflux disease, given that said symptomatology can be exacerbated during treatment with GLP-1 agonists.46,83
Included in the therapeutic measures for improving symptoms secondary to delayed gastric emptying are the dietary measures recommended for gastroparesis, such as a small particle size, fractionated diet containing < 40 g of fat and 10-12 g of fiber per day.84–86
Another recommendation is to evaluate the prescribed drug, given that adverse effects are dose-dependent and can present more often in patients that have not had progressive medication titration.52 Considering the type of GLP-1 agonist to be used, or that is being used, is important, because short-acting ones are associated with more episodes of nausea and vomiting than long-acting ones. Therefore, if a short-acting GLP-1 agonist is not well tolerated due to nausea and vomiting, treatment can be modified to a long-acting presentation, evaluating tolerance, before suspending this drug group.87–89
There is evidence on the prophylactic pharmacologic management of nausea and vomiting related to GLP-1 use. Ellero et al.90 reported that the administration of antiemetics prior to the subcutaneous application of exenatide was associated with fewer episodes of nausea and vomiting (16.7% and 6.7% vs 61.7% and 38.3%, p ≤ 0.001). The antiemetics analyzed were metoclopramide 10 mg and ondansetron 8 mg, administered as a single dose 30 minutes before applying the GLP-1 agonist.46,83
Regarding the pharmacologic management of nausea, vomiting, early satiety, and postprandial fullness induced by GLP-1 agonists, management aimed at pharmacologic gastroparesis or exacerbated functional dyspepsia could be offered, thus recommending the use of D2 dopaminergic receptor antagonists and 5HT4 antagonists. Of the abovementioned medications, metoclopramide stands out, with its antagonist effect on D2 dopaminergic and 5-HT3 serotoninergic receptors, as well as its agonist effect on 5-HT4 receptors. The recommended dose is 10 mg of oral metoclopramide every 8 hours, with preprandial dosing. The selective D2 dopaminergic antagonists that stand out are fast-acting domperidone at a preprandial dose of 10 mg every 8 hours and extended-release domperidone at a dose of 60 mg, given orally every 24 hours.46,51,79,83,91–93
Constipation associated with the use of GLP-1 agonists has a pathophysiologic mechanism secondary to the decrease in intestinal transit, colonic hyposensitivity, and probable increase in intestinal absorption of water. The recommendations for managing this type of secondary constipation are the same as those for slow transit chronic constipation or rectal hyposensitivity disorder.13,94,95 General measures, such as the use of defecation posture modification devices, having an adequate bowel movement routine obeying the defecatory urge, and not sitting on the toilet for long periods of time, drinking > 1.5 l of water per day, eating 25-30 g of hydrosoluble fiber per day or 14 g per 1,000 kcal, and having a breakfast that includes > 500 kcal, are recommended.94,96 Regarding pharmacologic management, osmotic laxatives are recommended as first-line management, and the drug of choice is polyethylene glycol at a dose of 17 g per day with titration. As second-line management, secretagogue laxatives are recommended. They include oral lubiprostone at a dose of 24 µg every 12 hours or oral linaclotide at a dose of 145 µg every 24 hours. The latter is preferred because lubiprostone is associated with nausea as an adverse effect. Another second-line pharmacologic option is prucalopride, a 5-HT4 serotoninergic agonist, with an established dose applied orally every 24 hours.94,97
Prucalopride is a good option in patients that experience adverse effects related to GLP-1 agonists because it increases gastric emptying, augmenting rectal sensitivity and intestinal transit. In a randomized controlled trial on patients with gastroparesis, Carbone et al.98 showed that prucalopride at a dose of 2 mg every 24 hours for 4 weeks improved the total Gastroparesis Cardinal Symptom Index (1.65 ± 0.19 vs 2.28 ± 0.20, p ≤ 0.0001) and also increased the mean time of gastric emptying measured by the 13C-octanoic acid breath test (98 ± 10 vs 143 ± 11 and 26 ± 13 minutes, p = 0.005 and < 0.001). There was improvement in the symptoms of nausea, early satiety, and postprandial fullness. A randomized controlled trial by Emmanuel et al.99 described evidence on the benefit of prucalopride in slow-transit constipation. A dose of 1 mg every 24 hours increased the frequency of spontaneous bowel movements (p ≤ 0.001) and intestinal transit (p = 0.004), as well as rectal sensitivity to distension (p = 0.01).
ConclusionsDiabetes is a chronic-degenerative disease with a high prevalence worldwide, conditioning high and costly morbidity and mortality. Adverse effects related to the use of antidiabetics are a large part of the morbidity and high costs in this population group. Antidiabetic-related gastrointestinal adverse effects are frequent and well established with the use of metformin, acarbose, and the GLP-1 agonists. Symptomatology depends on the underlying disease and the drug group utilized. The most frequent adverse effects are post-prandial distress syndrome, nausea, vomiting, bloating, diarrhea, and constipation.
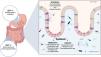
It is important to have the knowledge and understanding of the pathophysiologic mechanisms of these adverse effects, so that targeted and individualized decisions can be made, with respect to the diagnostic-therapeutic approach (Fig. 4).
There are preventive measures for avoiding the appearance of gastrointestinal symptoms, such as basing the choice of medication on the patient’s baseline symptoms, as well as prophylactic pharmacologic measures (Figs. 2 and 3). There are also specific therapeutic measures, according to the triggering disease, as exemplified by the use of prokinetics in patients with GLP-1 agonist-induced gastroparesis.
Nevertheless, it is important to identify alarm signs that warrant evaluation by experts in gastroenterology and the performance of an endoscopic examination, and not exclusively attribute symptoms to the use of antidiabetics.
Financial disclosureNo financial support was received in relation to this article.