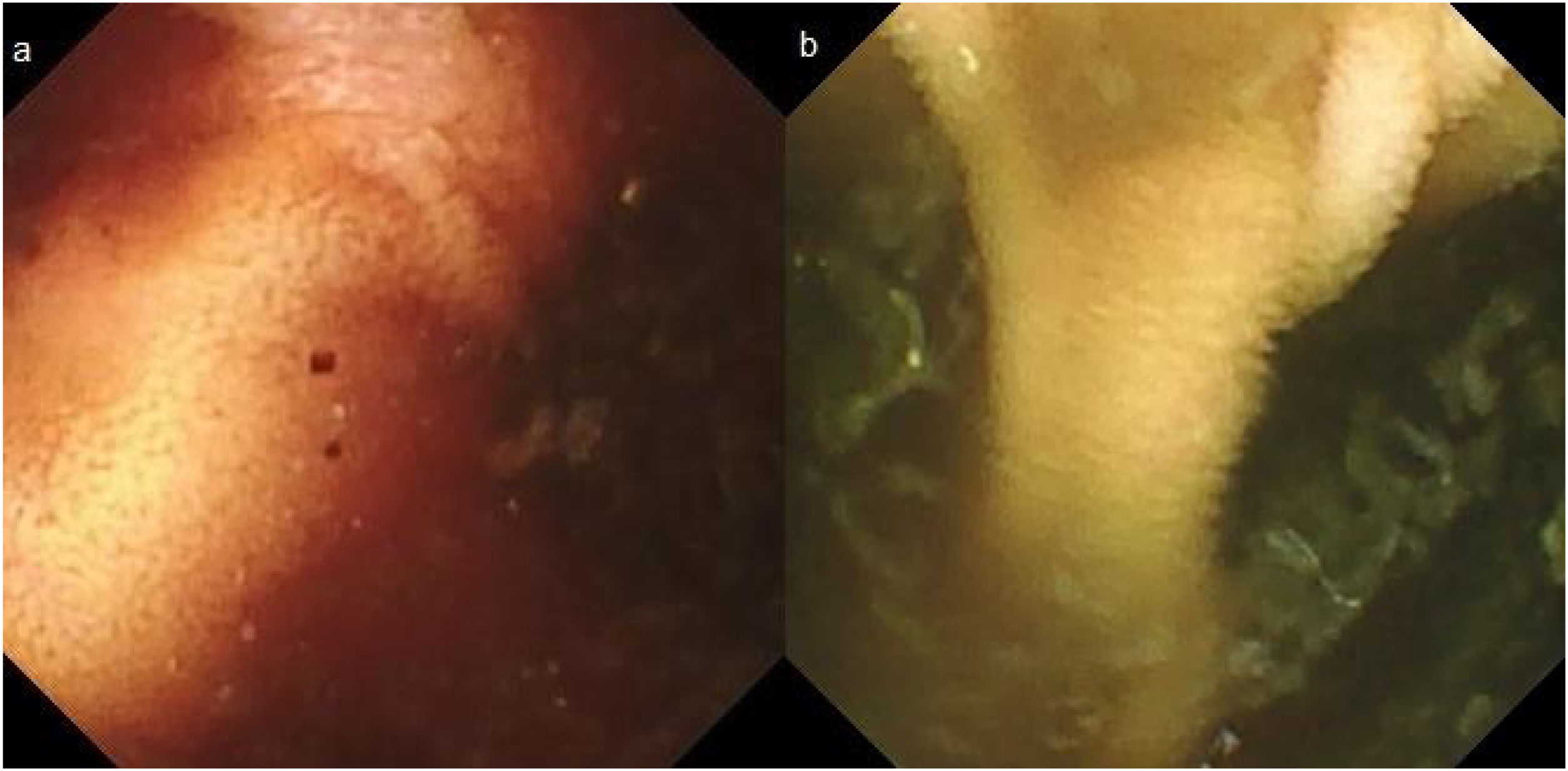
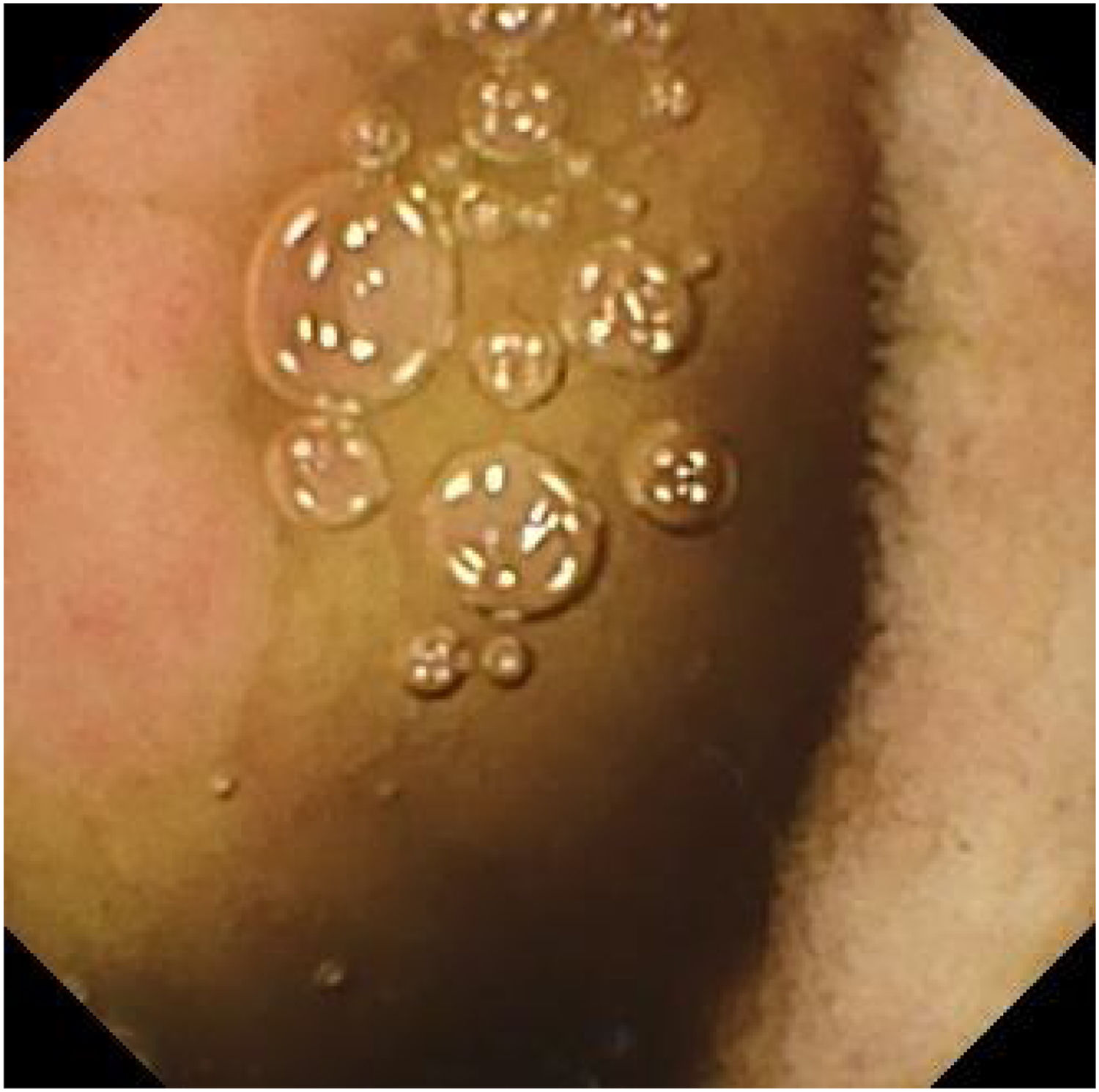
We present herein two cases: case 1 is a 71-year-old man who sought medical attention because of melenic stools. Complete blood count results were hemoglobin of 11.7g/dl (previously normal) and urea of 104mg/dl. After hemodynamic stabilization, upper endoscopy and colonoscopy were performed, finding no lesions. Capsule endoscopy (CE) revealed active bleeding in the jejunum, but its cause could not be visualized. The video capsule remained at the site, with an abundant content of blood, and could not advance. A double-lumen image (Fig. 1a) and atrophic mucosa with no villi were shown on the digital photos, suggesting a small bowel diverticulum. Plain abdominal x-ray confirmed that the video capsule was still in the intestine. The patient reported having no clinical symptoms of obstruction and the bleeding was self-limited. Five days after undergoing the CE, the patient again presented with melenic stools and hemodynamic instability. Abdominal computed tomography angiography (CTA) confirmed bleeding in the 4cm jejunal diverticulum that was retaining the video capsule. Laparotomy was performed, and a jejunal segment with large diverticula, one of which was retaining the video capsule, was resected. Case 2 corresponds to an 87-year-old man who sought medical attention due to hematochezia. Complete blood count results were hemoglobin of 9.9g/dl. Colonoscopy revealed diverticular disease of the left colon, with no signs of bleeding, and upper endoscopy was normal. CE was performed because of the persistence of bleeding that required transfusion (15 units of red blood cells). The video capsule remained in the jejunum more than 19h after the study, with no signs of bleeding in the segment examined. Digital photos showed a double-lumen image (Fig. 1b) and mucosa with no villi (Fig. 2), suggesting a jejunal diverticulum, albeit the abundant non-hematic intestinal content did not permit clear visualization. The patient did not present with signs of obstruction. Given the active bleeding, the evaluation of the patient was completed with a CTA, finding a 6cm jejunal diverticulum and active bleeding in the terminal jejunum and ileum. The video capsule was confirmed to have advanced into the transverse colon, and later, the bleeding point was percutaneously embolized.
CE is a safe procedure, with an incidence of complications below 1.5%. The main complication is video capsule retention, the risk of which is higher in patients with Crohn’s disease, anti-inflammatory drug use, intestinal tumors, radiation enteritis, or previous abdominal surgery1–4. A retention risk of up to 8.2% has been described in patients with Crohn’s disease4. The arbitrary definition of retention is when the video capsule is identified in an abdominal imaging study, 14 days or longer after having been swallowed.4 The majority of cases are asymptomatic and resolved conservatively. Unless there is high suspicion of a malignant etiology, conservative treatment of video capsule retention is recommended2,4. A published case report and literature review described a patient with video capsule retention for 4.5 years; it was finally retrieved endoscopically5.
In former clinical practice guidelines, diverticulosis of the small bowel and colonic diverticulosis were considered relative contraindications for CE1,6. However, there are few published cases of retention in that context2, and so current guidelines do not include them as contraindications3. Two cases were described herein, in which video capsule advancement was hindered by the presence of diverticula in the small bowel. In the first case, the capsule remained in the intestine for 5 days. It was then surgically retrieved, albeit the decision to operate was made, more with respect to the gastrointestinal bleeding than to the capsule retention. In the second case, even though capsule advancement was slower than expected, it spontaneously reached the colon. Nevertheless, our case series was small. Therefore, a larger number of cases and greater scientific evidence is needed to establish whether small bowel diverticula are a risk factor for incomplete examination of the small bowel and possible video capsule retention in CE.
Ethical considerationsThe authors declare that:
Informed consent was requested from the patients to receive treatment or participate in the research described.
The present work meets the current bioethical research regulations and was approved by the ethics committee of the Hospital Universitario Clínico San Cecilio.
This article contains no personal information that can identify the patients.
No experiments were conducted on animals or humans.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Díaz-Alcázar MM, Aguilar-Cruz I. ¿Es la diverticulosis intestinal un factor de riesgo para la cápsula endoscópica? Rev Gastroenterol Méx. 2022;87:390–392.