Helicobacter pylori (H. pylori) infection is usually acquired in childhood. It has not been widely studied in individuals within the pediatric age range in Cuba.
AimTo identify the prevalence of the infection and its associated risk factors in 3-year-old children in Havana.
Materials and methodsAn analytic, cross-sectional, epidemiologic analysis was conducted on 1,274 children belonging to the cohort of participants in the Natural History of Wheezing in Cuba study (HINASIC for its Spanish acronym) that were 3 years of age and provided a stool sample. H. pylori infection was identified by determining the H. pylori antigen (Ag) in stool, utilizing the commercial Spinreact kit, from Spain. The data were collected through a questionnaire applied by the researchers that included sociodemographic, environmental, and lifestyle variables, as well as infection from other parasites. Prevalence and the prevalence ratio with a 95% confidence interval were calculated and the dichotomous logistic regression analysis was employed.
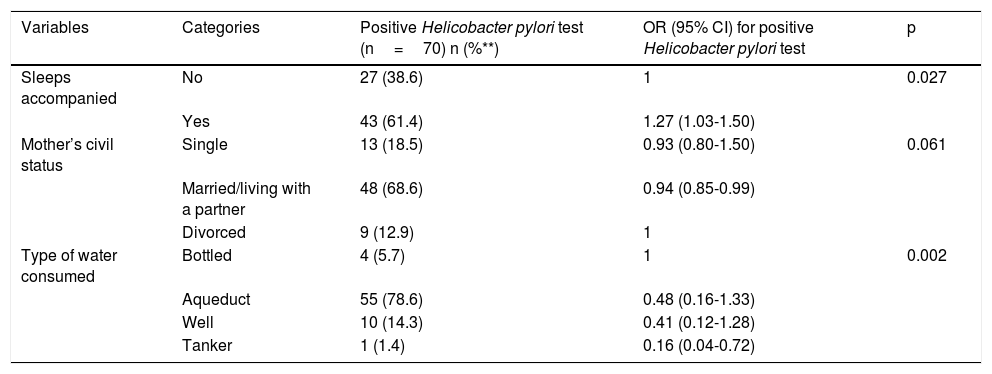
ResultsThe prevalence of positive H. pylori Ag was 5%. Sleeping together was the risk factor found (PR: 1.27; 95% CI: 1.03-1.50). Protective factors were drinking water from water delivery trucks (PR: 0.16; 95% CI: 0.04-0.72) and living in a nuclear family unit (PR: 0.94; 95% CI: 0.85-0.99).
ConclusionsThe prevalence of H. pylori infection in early childhood places Havana in an intermediate position at the international level. To control the infection, causal studies should be conducted and opportune interventions implemented.
La infección por Helicobacter pylori (H. pylori) es usualmente adquirida en la infancia. En Cuba su estudio en las edades pediátricas es un campo poco explorado.
ObjetivoIdentificar la prevalencia de la infección y los factores asociados en niños de 3 años de edad de La Habana.
Material y métodosSe realizó un estudio epidemiológico transversal analítico con 1274 niños de 3 años de edad, que aportaron muestra de heces, provenientes de la cohorte de nacimientos de La Habana (HINASIC). Se identificó la infección por H. pylori, utilizando el paquete para determinación de antígeno (Ag) de H. pylori en heces de la casa comercial Spinreact, España. La recolección de datos fue a través de un cuestionario administrado por los investigadores que incluyen variables sociodemográficas, ambientales, estilo de vida e infección por otros parásitos. Se calcularon prevalencias, razón de prevalencia con intervalos de confianza de 95% y regresión logística dicotómica.
ResultadosLa prevalencia de Ag de H. Pylori positivo fue de 5%. Dormir acompañado fue el factor de riesgo encontrado RP 1.27 (IC 95%: 1.03-1.50). Consumo de agua de pipa RP 0.16 (IC 95%: 0.04-0.72) y vivir en un núcleo familiar con ambos padres RP 0.94 (IC 95%: 0.85-0.99) fueron factores protectores.
ConclusionesLa prevalencia de infección de H. pylori en la infancia temprana ubica a La Habana internacionalmente en una posición intermedia. Estudios de causalidad e intervenciones futuras deberán ser tenidos en cuenta para el control de la infección.
Helicobacter pylori (H. pylori) colonization of the stomach is the most common of the chronic bacterial infections in humans.1 It is considered one of the main findings in gastroenterology and is one of the microorganisms of greatest interest in human pathology.2,3 Its origin dates back some 58,000 years.4 Between 1985-89, Warren and Marshall5 associated the presence of the bacterium with chronic gastritis and ulcer, and since then, much work has been carried out in that field. The genesis of chronic gastritis, duodenal and gastric ulcer in children and adults, and both gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma have been attributed to it. Once acquired, the infection persists, and the majority of infected individuals remain asymptomatic.
Calculations suggest that approximately half of the world’s population is colonized by H. pylori, with wide variability between different populations, possibly related to different factors, such as the virulence of the microorganism, host susceptibility, antibiotic use, and environmental conditions that include socioeconomic level. Genetic, racial, and cultural factors are less clearly involved.6,7 Transmission via the digestive route is well-accepted.6 Prevalence ranges from 1-2% in studies conducted on asymptomatic children in the Netherlands, to over 90% in Bangladesh.6 In developing countries, children are infected mainly before 10 years of age, and the highest prevalence peak in adults (80%) is before 50 years of age. In developed countries, studies show that infection is rarely produced before 10 years of age. It increases to 10% between 18 and 30 years of age and to 50% above 60 years of age.8
The prevalence of H. pylori infection in Cuba has not been widely analyzed. The majority of studies have been conducted on pediatric and adult populations with gastrointestinal symptoms. A high rate of H. pylori infection has been identified in adult patients with gastric ulcer (91%), duodenal ulcer (99%), and chronic gastritis (94%), with an elevated expression of the cagA+ gene.9–12 There are fewer studies on children, but a relation to anemia in school children (79%)13 and to diagnoses of antral chronic gastritis (67.7%) has been observed.14 Opportune preventive measures for stopping H. pylori transmission will reduce the incidence of those diseases and will depend on knowledge of the history of H. pylori infection. Therefore, the aim of the present study was to identify the prevalence of H. pylori infection and the associated socioeconomic, environmental, and lifestyle factors in the pediatric population of 3-year-old children in the province of Havana.
Materials and methodsStudy populationThe participants of the present analysis belong to the third year of the Natural History of Wheezing in Cuba (HINASIC for its Spanish acronym) study, a prospective, longitudinal, population study on children, whose aim was to identify risk factors for the development of asthma. Detailed information on the methodological design of the study, including sample selection, and the inclusion and exclusion criteria were previously described.15 A total of 1,543 three-year-old children who attended any of the 17 polyclinics of the 4 municipalities of Havana (Arroyo Naranjo, Cerro, Habana del Este, La Lisa) were interviewed within the time frame of March 2012 to March 2013. Of those children, 1,274 (83%) were eligible to participate in the present study by providing a stool sample.
Data collectionThe data were obtained through the application of a questionnaire administered by an interviewer (pediatricians or family physicians) to parents or guardians, after they had given their informed consent for their children to participate in the study. Information on demographics, lifestyle, and home environment, as well as specific questions on anthropometric measurements (weight and height) at the time of the interview, were collected. H. pylori infection was diagnosed from the stool sample provided by the participants, utilizing the commercial Spinreact kit (http://www.spinreact.com). As stated in the technical label, it is a qualitative immunochromatographic test that has 94% sensitivity and 95% specificity, compared with a reference standard. Test results were “positive” (presence of H. pylori IgG) or “negative” (absence of H. pylori IgG).16 Another portion of the stool sample was analyzed for parasitic infection, utilizing the direct smear method with Lugol’s solution and eosin staining, and the Kato-Katz concentration technique for identifying and quantifying helminth eggs.17
Data analysisThe information was collected in a database and obvious errors and non-plausible data were cleaned. The statistical analysis was performed using Stata v12 (StataCorp, Texas, USA) software and survey commands to allow the use of a planned sample design. Descriptive statistics were calculated (absolute frequencies, percentages) and a bivariate analysis was done using 2×2 contingency tables. The prevalence of infection and its 95% confidence interval were calculated in those tables for each exposure category of the variables, interpreting the statistical association when the interval did not contain the unit. The significant variables, and other scientifically plausible ones, were included in the modeling through dichotomous logistic regression. The result of the H. pylori test was the dependent variable, the probability that it was positive was modeled, and prevalence ratios and their 95% confidence intervals adjusted for each exposure variable were obtained, calculating robust standard errors adjusted by municipality. Sex and anti-parasitic treatment were considered a priori confounders but were not introduced into the final model because they did not modify the association by 10% or more.
Ethical considerationsWritten statements of informed consent were obtained from the parents or guardians of the underage patients for their participation in the study and the study protocol was approved by the scientific committees of the Instituto Nacional de Higiene, Epidemiología y Microbiología de La Habana and the University of Nottingham Medical School in the United Kingdom. The authors declare that no personal information that could identify patients is contained herein.
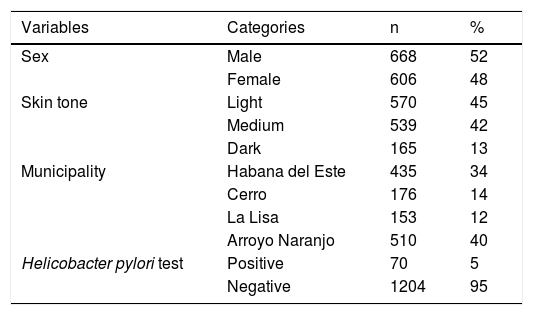
ResultsOf the 1,274 three-year-old children enrolled in the study, 100% of their parents or guardians correctly completed the questionnaire applied by the family physicians. The characteristics of the study participants are shown in Table 1. The prevalence of H. pylori infection was 5%.
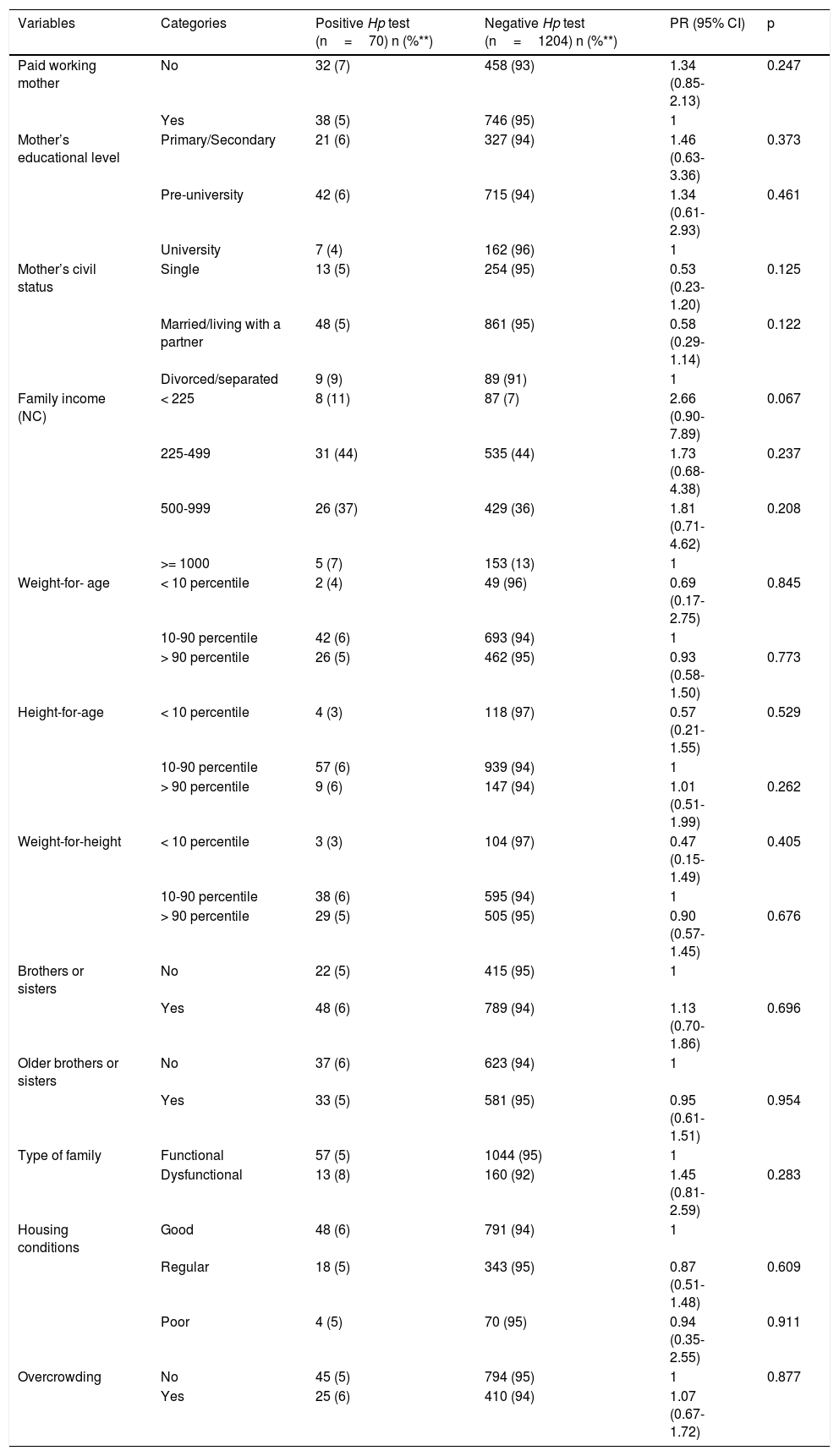
Table 2 shows the bivariate associations with H. pylori and Table 3 shows the factors associated with H. pylori infection, after the confounding factor adjustment. The risk factor found for H. pylori infection was sleeping together (PR 1.27; 95% CI: 1.03-1.50), and the protective factors were married/living with a partner (PR 0.94; 95% CI: 0.85-0.99) and drinking water delivered by a water truck (PR 0.16; 95% CI: 0.04-0.72).
Bivariate analysis of exposure to and risk for positive H. pylori Ag.
| Variables | Categories | Positive Hp test (n=70) n (%**) | Negative Hp test (n=1204) n (%**) | PR (95% CI) | p |
|---|---|---|---|---|---|
| Paid working mother | No | 32 (7) | 458 (93) | 1.34 (0.85-2.13) | 0.247 |
| Yes | 38 (5) | 746 (95) | 1 | ||
| Mother’s educational level | Primary/Secondary | 21 (6) | 327 (94) | 1.46 (0.63-3.36) | 0.373 |
| Pre-university | 42 (6) | 715 (94) | 1.34 (0.61-2.93) | 0.461 | |
| University | 7 (4) | 162 (96) | 1 | ||
| Mother’s civil status | Single | 13 (5) | 254 (95) | 0.53 (0.23-1.20) | 0.125 |
| Married/living with a partner | 48 (5) | 861 (95) | 0.58 (0.29-1.14) | 0.122 | |
| Divorced/separated | 9 (9) | 89 (91) | 1 | ||
| Family income (NC) | < 225 | 8 (11) | 87 (7) | 2.66 (0.90-7.89) | 0.067 |
| 225-499 | 31 (44) | 535 (44) | 1.73 (0.68-4.38) | 0.237 | |
| 500-999 | 26 (37) | 429 (36) | 1.81 (0.71-4.62) | 0.208 | |
| >= 1000 | 5 (7) | 153 (13) | 1 | ||
| Weight-for- age | < 10 percentile | 2 (4) | 49 (96) | 0.69 (0.17-2.75) | 0.845 |
| 10-90 percentile | 42 (6) | 693 (94) | 1 | ||
| > 90 percentile | 26 (5) | 462 (95) | 0.93 (0.58-1.50) | 0.773 | |
| Height-for-age | < 10 percentile | 4 (3) | 118 (97) | 0.57 (0.21-1.55) | 0.529 |
| 10-90 percentile | 57 (6) | 939 (94) | 1 | ||
| > 90 percentile | 9 (6) | 147 (94) | 1.01 (0.51-1.99) | 0.262 | |
| Weight-for-height | < 10 percentile | 3 (3) | 104 (97) | 0.47 (0.15-1.49) | 0.405 |
| 10-90 percentile | 38 (6) | 595 (94) | 1 | ||
| > 90 percentile | 29 (5) | 505 (95) | 0.90 (0.57-1.45) | 0.676 | |
| Brothers or sisters | No | 22 (5) | 415 (95) | 1 | |
| Yes | 48 (6) | 789 (94) | 1.13 (0.70-1.86) | 0.696 | |
| Older brothers or sisters | No | 37 (6) | 623 (94) | 1 | |
| Yes | 33 (5) | 581 (95) | 0.95 (0.61-1.51) | 0.954 | |
| Type of family | Functional | 57 (5) | 1044 (95) | 1 | |
| Dysfunctional | 13 (8) | 160 (92) | 1.45 (0.81-2.59) | 0.283 | |
| Housing conditions | Good | 48 (6) | 791 (94) | 1 | |
| Regular | 18 (5) | 343 (95) | 0.87 (0.51-1.48) | 0.609 | |
| Poor | 4 (5) | 70 (95) | 0.94 (0.35-2.55) | 0.911 | |
| Overcrowding | No | 45 (5) | 794 (95) | 1 | 0.877 |
| Yes | 25 (6) | 410 (94) | 1.07 (0.67-1.72) |
| Variables | Categories | Positive Hp test (n=70) n (%**) | Negative Hp test (n=1204) n (%**) | PR (95% CI) | p |
|---|---|---|---|---|---|
| Children’s circle or caretaker’s home attendance | No | 22 (6) | 349 (94) | 1 | |
| Yes | 48 (5) | 855 (95) | 0.90 (0.55-1.46) | 0.763 | |
| Sleeps accompanied | No | 27 (4) | 597 (96) | 1 | 0.095 |
| Yes | 43 (7) | 607 (93) | 1.53 (1.00-2.42) | 0.095 | |
| Treatment for other parasites | No | 60 (5) | 1115 (95) | 1 | |
| Yes | 10 (10) | 89 (90) | 1.98 (1.05-3.74) | 0.062 | |
| Treatment with antibiotics | No | 26 (6) | 406 (94) | 1 | |
| Yes | 44 (5) | 798 (95) | 0.87 (0.54-1.39) | 0.647 | |
| Pets | No | 43 (6) | 723 (94) | 1 | |
| Yes | 27 (5) | 481 (95) | 0.95 (0.59-1.51) | 0.918 | |
| Dogs | No | 51 (6) | 811 (94) | 1 | |
| Yes | 19 (5) | 393 (95) | 0.78 (0.47-1.30) | 0.410 | |
| Cats | No | 66 (5) | 1144 (95) | 1 | |
| Yes | 4 (6) | 60 (94) | 1.15 (0.43-3.05) | 1.000 | |
| Other animals | No | 59 (5) | 1080 (95) | 1 | |
| Yes | 11 (8) | 124 (92) | 1.57 (0.85-2.92) | 0.218 | |
| Rodents | No | 55 (6) | 926 (94) | 1 | |
| Yes | 15 (5) | 278 (95) | 0.95 (0.52-1.59) | 0.861 | |
| Vectors | No | 47 (5) | 831 (95) | 1 | |
| Yes | 23 (6) | 373 (94) | 1.08 (0.67-1.76) | 0.844 | |
| Type of water consumed | Bottled | 4 (12) | 29 (88) | 1 | |
| Aqueduct | 55 (6) | 910 (94) | 0.47 (0.17-1.26) | 0.124 | |
| Well | 10 (4) | 216 (96) | 0.37 (0.13-0.97) | 0.068 | |
| Tanker | 1 (2) | 49 (98) | 0.17 (0.03-0.69) | 0.059 | |
| Boils water | No | 8 (3) | 224 (97) | 1.73 (0.84-3.55) | 0.176 |
| Yes | 62 (6) | 980 (94) | 1 |
| Variables | Categories | Positive Hp test (n=70) n (%**) | Negative Hp test (n=1204) n (%**) | PR (95% CI) | p |
|---|---|---|---|---|---|
| Child’s habits | |||||
| Plays with soil or sand | No | 40 (5) | 797 (95) | 1 | |
| Yes | 30 (7) | 407 (93) | 1.43 (0.91-2.27) | 0.155 | |
| Eats soil or sand | No | 67 (5) | 1177 (95) | 1 | |
| Yes | 3 (10) | 27 (90) | 1.86 (0.62-5.57) | 0.490 | |
| Sucks his/her thumb | No | 56 (5) | 1028 (95) | 1 | |
| Yes | 14 (7) | 176 (93) | 1.43 (0.81-2.51) | 0.291 | |
| Bites/eats fingernails | No | 49 (5) | 873 (95) | 1 | |
| Yes | 21 (6) | 331 (94) | 1.12 (0.68-1.84) | 0.750 | |
| Walks barefoot on the ground | No | 48 (5) | 890 (95) | 1 | |
| Yes | 22 (7) | 314 (93) | 1.28 (0.76-2.09) | 0.400 | |
| Scratches his/her anus | No | 49 (5) | 910 (95) | 1 | |
| Yes | 21 (7) | 294 (93) | 1.30 (0.80-2.14) | 0.363 | |
| Washes hands after urinating/defecating | No | 18 (7) | 252 (93) | 1 | |
| Yes | 52 (5) | 952 (95) | 0.78 (0.46-1.31) | 0.423 | |
| Washes hands before eating | No | 7 (5) | 130 (95) | 1 | |
| Yes | 63 (6) | 1074 (94) | 1.08 (0.51-2.32) | 0.991 | |
| Washes hands after playing with soil or sand | No | 17 (5) | 296 (95) | 1 | |
| Yes | 53 (6) | 908 (94) | 1.02 (0.60-1.73) | 1.000 | |
| Other parasitic infections | |||||
| Parasitic infection | No | 5 (14) | 1172 (86) | 1 | |
| Yes | 65 (5) | 32 (95) | 2.57 (1.10-6.01) | 0.071 | |
| Helminthiasis | No | 69 (5) | 1200 (95) | 1 | |
| Yes | 1 (20) | 4 (80) | 3.68 (0.63-21.6) | 0.658 | |
| Protozoa | No | 4 (13) | 1176 (87) | 1 | |
| Yes | 66 (5) | 28 (95) | 2.35 (0.91-6.06) | 0.171 | |
95% CI: 95% confidence interval; PR : Prevalence ratio.
%*: Percentage with respect to the total number of children (n=1274).
%**: Percentage with respect to the total per exposure category.
The p values were obtained from association tests based on the X2 distribution.
Multivariate analysis of exposure to and risk for positive H. pylori Ag.
| Variables | Categories | Positive Helicobacter pylori test (n=70) n (%**) | OR (95% CI) for positive Helicobacter pylori test | p |
|---|---|---|---|---|
| Sleeps accompanied | No | 27 (38.6) | 1 | 0.027 |
| Yes | 43 (61.4) | 1.27 (1.03-1.50) | ||
| Mother’s civil status | Single | 13 (18.5) | 0.93 (0.80-1.50) | 0.061 |
| Married/living with a partner | 48 (68.6) | 0.94 (0.85-0.99) | ||
| Divorced | 9 (12.9) | 1 | ||
| Type of water consumed | Bottled | 4 (5.7) | 1 | 0.002 |
| Aqueduct | 55 (78.6) | 0.48 (0.16-1.33) | ||
| Well | 10 (14.3) | 0.41 (0.12-1.28) | ||
| Tanker | 1 (1.4) | 0.16 (0.04-0.72) |
The p values were obtained from Wald significance tests based on X2 distribution.
Definitive model: PY=1=11+e--3.1465+0.2342*SleepsAccompanied-0.2492*SingleMother-0.1776*MarriedMother+0.1164*AqueductWater-0.0535*WellWater-0.9912*TankerWater
To the best of our knowledge, the present analysis is the first population-based study investigating the prevalence of and factors associated with H. pylori infection in a population of healthy 3-year-old children in the capital city of Havana, Cuba. The prevalence of bacterial infection in the study population was the result of environmental and lifestyle conditions. The cross-sectional analysis conducted on the 3-year-old children appearing in a database of the longitudinal HINASIC cohort provided the knowledge of the magnitude of the infection at that age. Sleeping accompanied was an important risk factor and the civil status of married/living with a partner and drinking water from a water-delivery truck were protective factors.
From the total number of 3-year-old children surveyed, 83% provided the stool samples needed for H. pylori testing, which enabled the prevalence of the infection to be calculated. The strengths of our study were the fact that the questionnaires were correctly formulated and applied by qualified medical personnel that had been following the children for several years, the majority of questionnaires were answered by the children’s parents (90%), and an internationally validated H. pylori test recommended for epidemiologic studies on that age group was employed.18 In addition, highly qualified technical personnel from the Instituto Nacional de Higiene, Epidemiología y Microbiología de Cuba (INHEM) carried out the external control of the results of the parasitology tests performed at the parasitology laboratories of the health areas involved, guaranteeing the reliability of the stool test results. The following two limitations of our study were identified: a history of ulcers or gastritis in the parents or guardians of the participating children was not determined and thus could not be analyzed as an associated factor, and the low prevalence of H. pylori Ag made it difficult to analyze the possible associated factors. Nevertheless, the results are valuable.
Cuba is a country that has medium and low resource settings. Despite those conditions and the strong economic blockade imposed by the United States for more than 50 years, unlike other countries with the same level of development, its health and education indicators are similar to those of the developed world. That situation, the fact that our study was conducted in an urban zone, and the high rate of antibiotic use in the pediatric population most likely were contributing factors to our study results.19–22 The prevalence of H. pylori in the 3-year-old children analyzed places us in an intermediate prevalence pattern in the international context, but we are close to the pattern of industrialized countries, if we take into account that early childhood prevalence is approximately 1.2% in Western Europe.6 In the Latin American countries of Ecuador, Chile, Brazil, and Mexico, an important percentage of children are already infected (63%, 25%, 31.1% and 10%, respectively).21,23–26 On the African continent, studies by Amberbir et al. describe a prevalence of 41% in children 3 years of age in an Ethiopian population, and in Uganda, 28.7% in 1-year-old children, 46% of children between 1 and 2 years of age, and 51.7% of children between 3 and 5 years old are already infected.27,28 In a recent meta-analysis conducted by Zabala Torres et al., those authors report prevalence of 20% in children under 6 years of age (95% CI: 14%-25%), among other results.29
Children sleeping together with other persons was the risk factor identified in the present study. Regardless of not having determined a history of infection in the adults living with the children, a high prevalence of infection detected in the adult Cuban population (75-90%)8–12,14 and evidence of infection transmission between couples suggest an increased risk for transmission to children living with infected individuals.24,25,29–32 Family has been found to play a fundamental role in the transmission of the H. pylori bacterium, especially within the first years of life, during which the most probable cause is oral-oral transmission from the mother to the child.24,29,32
It appears that living in a stable nuclear family unit is a protective factor against bacterial infection. Greater care given to the children is most likely an important factor and a plausible explanation is the presence of better personal and environmental hygiene habits related to a higher educational level of the study population. In addition, the greater antibiotic use detected in the study participants was relevant, which could lend important weight to that result.24,31
The consumption of potable water delivered by water truck (tankers) was another factor that contributed to the protection against H. pylori infection. The technical condition of the water supply system is not good in all parts of Havana and therefore the distribution of water by tankers is necessary. Despite the inconvenience of that type of public service, it appears that water is safer for drinking, if it does not flow through the water pipeline network, where it can become cross-contaminated due to structural deficiencies. In their 1997 study, Johnson et al. showed that chlorinated water was a factor that inactivated the bacterium, interfering with its transmission by water.33 A recent review revalidated contaminated water as a source of H. pylori, but insists on the need for further detailed study on that route of transmission.34 Eichelberger et al. presented information on the association between environmental risk factors and H. pylori infection in the United States based on data from the National Health and Nutrition Examination Survey (NHANES), indicating that environmental exposure (incorrect water use and occupational contact with soil) play an important role in the transmission of H. pylori (adjusted odds ratio [OR] 2.7, 95% CI: 1.3-5.6).35
In conclusion, the estimated prevalence of H. pylori infection in early childhood places Havana in an intermediate position in the international context. Socioeconomic conditions appear to be important factors in the presence of the infection. Opportune interventions should be implemented to reduce future complications.
Financial disclosureThe present study was funded by the Wellcome Trust (090375), the Nottingham University Hospital Charitable Trust, the Nottingham Respiratory Biomedical Research Unit, and the Instituto Nacional de Higiene, Epidemiología y Microbiología, Havana, Cuba.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the Natural History of Wheezing in Cuba (HINASIC, the Spanish acronym) study group, made up of the following members:
Instituto Nacional de Higiene, Epidemiología y Microbiología
Silvia J Venero-Fernández, Ramón Suárez-Medina, Hermes Fundora-Hernández, Lenina Menocal-Heredia, Yuria Isabel Caraballos-Sánchez, Reina Amelia Quintana, Félix Manuel Rosado-García, Patricia Varona-Pérez, María del Carmen Hinojosa.
Hospital Universitario Pediátrico Docente Centro Habana
Regla N. Rivero, Javier Muñoz-Pérez, Caridad González-Morfa.
Municipio de Arroyo Naranjo
Esperanza de la C. Mora-Faife, Damaris Zaldívar-Ricardo, Maritza Diburt-Amita, Gisela Álvarez-Valdez, Anadelis Alfonso-Hernández, Vilma Álvarez-Valdez, Yamilka Magaña-Álvarez, Zoe de los Ángeles Figueroa-Barreto, Nieves Sardiñas-Báez, Jorge Antonio Febles-del-Toro, Yunia Velázquez-Pérez, Manuel Hugo Felpeto-Fuentes, Yordanka Gainza-Bueno, Grisel M. Esquivel-Barrios, Maite Suárez-Paz, Bárbara Judith Magaña-Álvarez, Alida Carménate-Fernández, Roberto Hidalgo-Mederos, Lianet del Loreto, Hidalgo Mederos, Danay Silva, Gretel Comas Fonseca, Dalia M Lazaga Cala, Cristina Odalys, Kessel Díaz, Aliuska Lorenzo Méndez
Municipio de La Lisa
Gladys García-García, María de Lourdes Ortiz-Hernández, María Antonia Betancourt-López, Marlén Batista-Cedeño, Iris Alfonso-Castellanos, Leticia Gómez-García, Ernesto Rafael Gutiérrez-Mendoza, María Luisa Loynaz-González, Nibenia Rodríguez-Trujillo, Yanet Pozo-Herrera, Víctor Manuel Montejo-Guerra, Julia Urbina-Reynaldo, Valentina Gómez-Suliman, Caridad Alicia Rodríguez-Aragón.
Municipio de Cerro
Ileana del Valle-Infante, Claudia Matos-Ramos, Martha Betancourt-Orue, Oscar Alba-Monteagudo, Yuderkis Ferrer-Ceruto, Aída Damas-Martínez, Mercedes Peñalver-Pérez
Municipio de Habana del Este
Liem Gómez-Marrero, Sarahí Castillo-Martínez, Amor de los Angeles Castaño-Vega, Norberto Torriente-Barzaga, Ileana Ávila-Rodríguez, Magalys Navarro-Ruiz, Kirenia Díaz-Hernández, Iluska De La Torre Suarez, Gilberto Roque-Pereira, Yamilet Corona-Carnero, Idania Gonzalaz-Fernandez, Fidelia Romeu-Ravelo, Regla Hernandez-Ponce, Teresa Serrano-González, Dulce Romeo-Cepero, Caridad Gonzalez Leiva, Teresa De Jesus Cobas Espino, Nuris-Fajardo, Midiala Perez-Arcia, Sarahy Diaz-Araujo, Yanet Medina- Lescay, Sandra Collazo-Rodriguez, Julia Amparo Griñán-Ramos, Teresa Serrano-Gonzalez, Beatriz Lazo-Vazquez, Tania Pupo-Portal, Nidia Leyva-Porra, Odalys Pacheco-Mesa, Martha Rizo-Ramos, Yaneysi Vallafuerte-Perez, Aliniuska De La Paz-Arias, Maite B Garcia-Sotolongo, Yusimí Calzado-Herrera, Martha Nidia Rizo-Ramos, Guillermo Verdecia, Mayté B García-Sotolongo, Juana F Abreu-Quijano, Fidelia Romeo-Ravelo.
The authors also wish to thank the children and parents for providing their data and the municipal Public Health directors and laboratory personnel that supported the study.
Please cite this article as: Venero-Fernández SJ, Avila-Ochoa I, Menocal-Herredia L, Caraballo-Sánchez Y, Rosado-García FM, Suárez-Medina R, et al. Prevalencia y factores asociados a infección por Helicobacter pylori en preescolares de La Habana, Cuba. Estudio de base poblacional. Revista de Gastroenterología de México. 2020;85:151–159.