Sprue-like enteropathy due to olmesartan produces symptoms of diarrhea and weight loss, as well as intestinal histologic changes associated with its use. The entity was described in 2012 in a study on 22 patients.1 In 2013, the Food and Drug Administration (FDA) released an alert about the relation between olmesartan use and enteropathy, and numerous cases have been reported since then.2
We present herein the case of a 74-year-old woman, with chronic ischemic heart disease and multiple cardiovascular risk factors, that was admitted to our center due to the sudden onset of diarrhea of 3-week progression. Stools were watery, with no pathologic product, and the patient presented with more than 10 evacuations daily. There were no other accompanying clinical manifestations. Symptoms resolved spontaneously after 48 h of hospital admission and the patient was released. Five days later, the patient returned, complaining of the same symptoms. They again resolved after 24 h of conservative hospital treatment and the patient was discharged to her home. Forty-eight hours after her second release, the patient was admitted to the intensive care unit, presenting with new episodes of diarrhea, hypotension that was refractory to fluid therapy, and acute kidney failure. She received support treatment, without requiring vasoactive drugs, and the diarrhea resolved after the first 72 h.
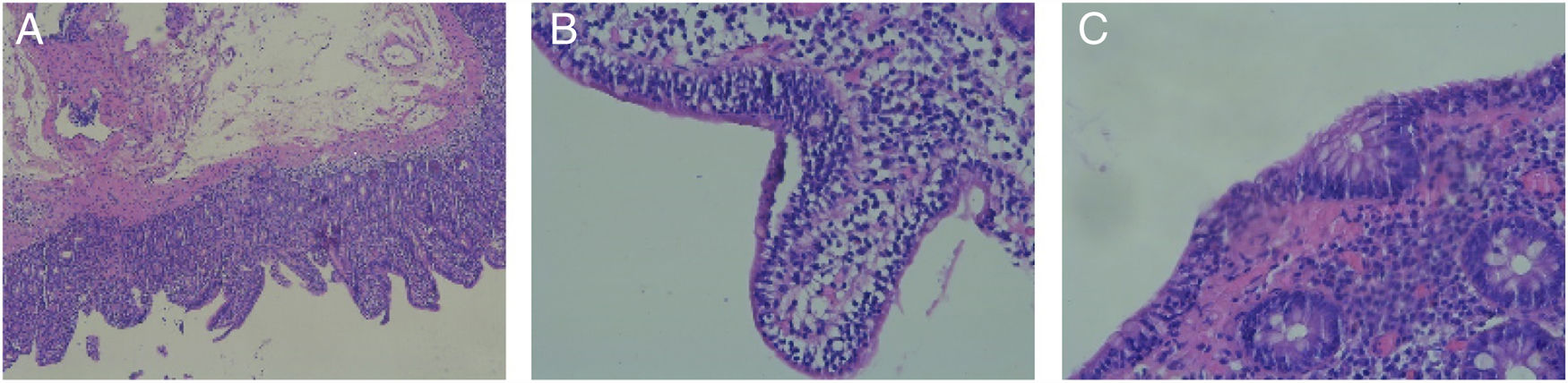
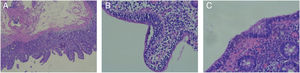
Adult celiac disease testing was negative, with normal IgA levels and negative results for IgA anti-tissue transglutaminase antibodies. Gastroscopy and colonoscopy were performed, and the histologic findings were villous atrophy, intraepithelial lymphocytes, and crypt hyperplasia, consistent with Marsh classification grade 3a (fig. 1A and B). Biopsy of the colon revealed intraepithelial lymphocytosis (Fig. 1C). Those findings were consistent with the histology described for olmesartan-induced sprue-like enteropathy.3
Finally, given the case progression, and after reviewing the literature, treatment with olmesartan was suspended; the drug had not been administered during the patient’s hospital admissions because it was not available at our hospital center’s pharmacy. Steroid treatment (methylprednisolone 16 mg/12 h) was begun, symptoms improved, and the patient was discharged to her home, with tapering of the corticosteroid therapy. At month six of follow-up, the patient has not presented with new episodes of diarrhea.
The real incidence of olmesartan-induced sprue-like enteropathy is currently unknown, albeit there are an increasing number of reported cases. The previously undescribed particularity observed in the progression of our patient was its severity, resulting in her admission to the intensive care unit. Symptom response to the suspension of olmesartan, as well as to corticoid treatment, which is the only management alternative described once the clinical presentation has occurred, should also be emphasized.4 Nevertheless, maintaining a high level of suspicion regarding the pathology appears to be the most important aspect, to prevent the performance of unnecessary studies and the prolongation of symptoms that can become severe, as seen in our patient.
In conclusion, olmesartan-induced sprue-like enteropathy is an emerging pathology that has begun to be diagnosed exponentially, since the detection of the first cases, and should be kept in mind in the differential diagnosis of diarrhea. In addition, it exemplifies the important role of drugs as etiologic factors of numerous pathologies, reminding us not to ignore them as possible causes.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have treated all patient data with confidentiality and anonymity, following the protocols of their work center. Because our center’s treatment protocol for this pathology was followed, authorization from the bioethical committee was not required.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Alonso-Beato R, González-Pérez JA, Demelo-Rodríguez P. Enteropatía sprue-like grave por olmesartán con buena respuesta a tratamiento esteroideo. Revista de Gastroenterología de México. 2020;85:220–222.