Patients with disorders of the gut-brain axis, such as irritable bowel syndrome (IBS), often exhibit small intestinal bacterial overgrowth (SIBO). Its treatment includes rifaximin (RF), ciprofloxacin (CF), neomycin, sulfamethoxazole-trimethoprim, and metronidazole (MZ). RF is a non-absorbable antibiotic, postulated to have fewer adverse effects. Our aim was to assess symptomatic response and SIBO eradication in patients with IBS, using three antibiotic regimens.
MethodsA prospective, randomized, double-blind study was conducted on IBS patients over 18 years of age, utilizing the Rome IV questionnaire and lactulose breath test. Those diagnosed with SIBO were randomly assigned to receive antibiotic treatment. Group A was treated with RF, group B with CF, and group C with MZ, each for 10 days. Treatment response was evaluated based on the SIBO eradication rate 15 days after completing therapy, utilizing hydrogen and methane breath tests with lactulose. Self-reported symptoms were recorded on a 10-point Likert scale before, during, and after treatment.
ResultsNinety-seven patients with IBS and SIBO were included, 81% of whom completed treatment. Fifty-nine percent of the patients treated with RF achieved SIBO eradication, compared with 53% and 79% of those treated with CR and MZ, respectively. Metronidazole reduced more methane levels, compared with the other groups. However, the greatest reduction in abdominal pain and bloating was observed in the RF group, with a lower percentage of adverse events.
ConclusionsPatients with IBS and SIBO benefit from antibiotic therapy. MZ exhibited the best SIBO eradication rate, but RF demonstrated greater symptomatic improvement and a lower rate of adverse effects.
Los pacientes con trastornos del eje intestinos-cerebro, como el síndrome de intestino irritable (SII), a menudo muestran sobrecrecimiento bacteriano en intestino delgado (SBID). Su tratamiento incluye rifaximina (RF), ciprofloxacino (CF), neomicina, sulfametoxazol-trimetoprima y metronidazol (MZ). La RF es un antibiótico no absorbible, que se ha observado que tiene pocos efectos secundarios. Nuestro objetivo fue evaluar la respuesta sintomática y la erradicación del SBID en pacientes con SII, utilizando tres regímenes antibióticos.
MétodosSe realizó un estudio de doble ciego, aleatorizado y prospectivo con pacientes con SII mayores de 18 años, utilizando el cuestionario de Roma IV y la prueba de aliento con lactulosa. Los pacientes diagnosticados con SBID fueron asignados aleatoriamente para recibir tratamiento con antibiótico. El grupo A recibió tratamiento con RF, el grupo B con CF y el grupo C con MZ, cada uno durante 10 días. La respuesta al tratamiento fue evaluada con base en la tasa de erradicación del SBID 15 días después de completar la terapia, utilizando pruebas de aliento con hidrógeno y metano con lactulosa. Los síntomas autoreportados fueron registrados en una escala de Likert de 10 puntos, antes, durante y después del tratamiento.
ResultadosSe incluyó a 97 pacientes con SII y SBID, de los cuales el 81% completó el tratamiento. El 59% de los pacientes tratados con RF logró la erradicación del SBID, contra 53% y 79% de los pacientes tratados con CR y MZ, respectivamente. En el grupo de metronidazol los niveles de metano disminuyeron más que en los otros dos grupos. Sin embargo, la mayor disminución en dolor abdominal e inflamación fue observada en el grupo de RF, con un menor porcentaje de eventos adversos.
ConclusionesLos pacientes con SII y SBID se benefician de la terapia con antibióticos. El MZ mostró la mejor tasa de erradicación de SBID, pero la RF mostró una mejoría sintomática más importante y una menor tasa de eventos adversos.
Disorders of the gut-brain axis (GBA) are defined as alterations in brain-intestine-gut microbiota communication, which manifest as chronic and recurrent digestive symptoms. Irritable bowel syndrome (IBS) is the most common and is defined according to the Rome IV criteria as chronic abdominal pain associated with changes in bowel habits, defecation patterns, or both, lasting for a minimum of 12 weeks in the last 6 months.1 IBS accounts for 28% of gastroenterology consultations in the United States. Locally, a randomly applied Rome II criteria survey in Santiago, Chile, reported a similar incidence of 29% for IBS and 64% for digestive symptoms compatible with a functional disorder.2
The origin of symptoms is multifactorial, and various pathophysiologic mechanisms have been demonstrated in these patients. They include the presence of small intestinal bacterial overgrowth (SIBO), which has been described in up to 78%.3–7 The diagnosis of SIBO is defined as the presence of more than 10^3 colony-forming units (CFUs) in the lumen of the small intestine, determined through jejunal aspirate cultures.4,8,9 However, there are less invasive indirect techniques that detect products of bacterial metabolism in exhaled air, such as the production of hydrogen and methane, in response to various carbohydrates (lactulose, xylose, or glucose).9–11
SIBO produces symptoms that primarily include altered bowel habits, bloating, abdominal pain, and infrequently, malabsorption in more severe cases, which may overlap with symptoms of other disorders of the GBA. In the presence of SIBO, clinical guidelines suggest targeting therapeutic strategies at possible causes, which have been reported to include chronic constipation, chronic liver damage, obesity, and systemic sclerosis, among others.12 There is evidence in the literature that antibiotic treatment improves symptoms in these patients, once SIBO is eradicated, especially in recurrent cases, attributed to the presence of dysbiosis in the small intestine.13
Multiple antibiotics are recommended for the treatment of SIBO, including metronidazole and ciprofloxacin, both with a response rate close to 50%.14 Rifaximin use is currently increasing in our medical environment. It is a non-absorbable, broad-spectrum antibiotic, administered orally, that has proven to be effective in treating both aerobic and anaerobic Gram-positive and Gram-negative microorganisms, with a favorable safety profile. Some studies have reported an eradication rate of 84% for SIBO and overall symptom improvement ranging from 33% to 92%, in patients with IBS.15 However, in our environment, the response rate to these antibiotics and their oral tolerance, with respect to IBS-associated symptoms, is unknown. Therefore, our aim was to compare rifaximin use with that of metronidazole and ciprofloxacin, as treatment for SIBO in patients with IBS, and to determine its effect on reducing digestive symptoms.
MethodsA prospective, longitudinal, randomized, double-blind study was conducted on patients above 18 years of age, who presented a clinical picture compatible with IBS, according to the Rome IV criteria.1
The CONSORT checklist was employed. SIBO diagnosis was confirmed through a hydrogen and methane breath test with lactulose at the Functional Diseases Laboratory of the Clinical Hospital of the University of Chile. The Rome IV questionnaire for functional gastrointestinal disorders, validated in Spanish, was used for diagnosing IBS.2 Patients were blindly randomized into 3 groups: receiving oral treatment with rifaximin-α 400 mg every 12 hours for 10 days (group A), ciprofloxacin 500 mg every 12 hours for 10 days (group B), or metronidazole 500 mg every 8 hours for 10 days (group C). Patients received a kit containing the dose of the corresponding treatment indicated only by a number, together with a symptom survey and an adverse event report. The blind was lifted once all 97 kits were completed, and the control hydrogen and methane breath test with lactulose was performed. The exclusion criteria were antibiotic use in the last 12 weeks and/or prokinetic use in the last 15 days; patients with other gastrointestinal or cardiovascular diseases, diabetes, nephropathy, or cirrhosis; prolonged use of cardiotonic drugs; pregnant or breastfeeding women; and individuals or a family member with a known allergy to any of the antibiotics to be used.
Hydrogen and methane breath test with lactuloseAll enrolled patients underwent a hydrogen and methane breath test, with lactulose as the substrate, using a standardized technique for 180 minutes,8,9,15,16 at the beginning of the study and then 15 days after completing the antibiotic treatment. Patients were required to fast for 12 hours, follow a low-carbohydrate diet 48 hours before the test, refrain from using antibiotics for 15 days prior to the exam, and avoid bowel preparation for colonoscopy one month prior. Baseline exhaled air samples were collected, using a syringe connected to a nozzle at the end of a normal expiration, and then every 10 minutes, until completing 180 minutes. This was done following the ingestion of 10 g of lactulose dissolved in 200 ml of distilled water. The samples were analyzed using a gas chromatograph (Quintron BreathTracker®, USA), and the results were expressed in parts per million (ppm). A positive test for SIBO was considered when there were 2 or more readings > 20 ppm of H2 or > 10 ppm of CH4 above the baseline values, during the first 60 minutes, or readings with values above 20 ppm from the baseline.15
In addition, orocecal transit time (OCTT) was evaluated, corresponding to the time elapsed between the ingestion of lactulose and the onset of curve elevation after 60 minutes (normal reference range 80-100 minutes). Said time reflects the metabolism of lactulose by the normal bacterial flora of the colon. In the presence of SIBO, two elevations in the curve from the baseline are observed: an early elevation caused by the degradation of lactulose by bacteria in the small intestine, and a second sustained elevation determined by the OCTT.
Evaluation of clinical response to therapy and adverse eventsA self-reported daily symptom survey was conducted at the beginning of the study, during the study, and after completing the 10-day treatment. It assessed the intensity of pain, bloating, or meteorism; the number of bowel movements per day; and stool consistency based on the Bristol Stool Scale. Symptoms were reported using a Likert-type intensity scale from 0 to 10. The symptoms and drug-related adverse events were recorded during antibiotic intake, noting the presence of digestive symptoms, such as nausea, burning epigastric pain, heartburn, a metallic taste in the mouth, and vomiting. Additionally, extra-digestive symptoms, such as headache, joint pain, allergic rash, and fever, were assessed.
Statistical analysisThe treatment response rate comparing the 3 groups was evaluated using the chi-square test. The baseline and post-treatment H2 and CH4 levels and OCTT were compared across the 3 treatments, utilizing the non-parametric Kruskal-Wallis test, after the D’Agostino-Pearson normality test. Pre-treatment and post-treatment H2 and CH4 levels during the control hydrogen test were assessed, using a mixed-effects model (two-way ANOVA) with a Sidak post-test for multiple comparisons. Symptom reduction was evaluated in the 3 groups of patients, using a linear regression model, by calculating symptom slopes, during and after treatment. The association between drug use, the occurrence of adverse effects, and the SIBO response rate was evaluated using the Baptista-Pike test for the odds ratio. A result was considered statistically significant with a p value < 0.05.
Ethical considerationsPatients were asked to provide informed consent for receiving the treatment, participating in the above-described research. No minors were included. The study was approved by the Scientific Ethics Committee of the Clinical Hospital of the University of Chile, with ethics approval number OAIC N°546/16. The authors declare that this article contains no personal information that could identify the patients.
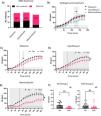
ResultsEradication of small intestinal bacterial overgrowth, in response to different treatment regimensNinety-seven patients were enrolled in the study: 32 in group A, 32 in group B, and 33 in group C. Table 1 describes the demographic and clinically relevant characteristics of the patients. Of the 97 patients, 41 (42%) met the criteria for IBS-D, 36 (37%) met the criteria for IBS-C, and 20 (21%) were considered IBS-M. They were randomly assigned to the 3 treatment groups, with no differences between them. Of the total number of patients, 27, 28, and 24 patients in groups A, B, and C, respectively, completed the study, with no differences in terms of age, sex, or type of IBS. Regarding SIBO eradication (Table 1), 79% of the patients that received metronidazole had a control lactulose test without SIBO, compared with 59% in the rifaximin group and 54% of those treated with ciprofloxacin (Fig. 1A). When comparing H2 levels in the post-treatment breath test, metronidazole significantly reduced H2 levels, compared with rifaximin and ciprofloxacin, at measurements taken at 120, 130, and 140 minutes (p value = 0.04, 0.03, 0.04, respectively) (Fig. 1B), with no differences in the remaining times. There were no differences in CH4 reduction between the 3 groups, post-treatment (Supplementary Fig. S1A), or in the pre-treatment H2 and CH4 levels (Supplementary Fig. S1B and C).
Patient clinical characteristics, treatment efficacy, and adverse effects. The patients were blindly randomized into 3 groups, receiving oral treatment with rifaximin 400 mg every 12 hours for 10 days (group A), ciprofloxacin 500 mg every 12 hours for 10 days (group B), or metronidazole 500 mg every 8 hours for 10 days (group C).
| Group A | Group B | Group C | ||
|---|---|---|---|---|
| Patients at the beginning of the study | 32 | 32 | 33 | |
| Patients at the completion of the study | 27 | 28 | 24 | |
| Patients lost to follow-up | 5 | 4 | 9 | |
| Median age (range) | 43 (20–68) | 42 (18–66) | 47 (20–66) | |
| Sex (M:F) | (2:25) | (0:27) | (3:21) | |
| BMI | 26 (19–46) | 25 (17–32) | 25 (17–37) | |
| Baseline OCTT (min) | 80 (70–150) | 90 (70–140) | 85 (70–170) | |
| Post-treatment OCTT (min) | 80 (60–170) | 80 (60–120) | 70 (60–130) | |
| Baseline H2 (ppm) | 16.56 ± 23.17 | 12.33 ± 15.09 | 21.42 ± 18.64 | |
| Post-treatment H2 | 11.04 ± 14.89 | 11.63 ± 13.78 | 8.04 ± 13.92 | |
| Baseline CH4 | 9.89 ± 10.28 | 9.3 ± 10.44 | 10.83 ± 8.93 | |
| Post-treatment CH4 | 9.81 ± 10.11 | 6.44 ± 7.02 | 6.83 ± 10.09 | |
| Post-treatment negative SIBO n (%) | 16 (59%) | 15 (54%) | 19 (79%) | |
| Post-treatment positive SIBO n (%) | 11 (41%) | 13 (46%) | 5 (21%) | |
| Adverse effects | Based on the total number of patients per group | |||
| Total | N(%) | 3 (9%) | 13 (41%) | 13 (40%) |
| Nausea | 0 | 4 (12%) | 6 (18%) | |
| Vomiting | 1 (3%) | 1 (3%) | 1 (3%) | |
| Diarrhea | 0 | 1 (3%) | 2 (6%) | |
| Allergy/rash | 0 | 2 (6%) | 1 (3%) | |
| Headache | 2 (6%) | 4 (12%) | 3 (9%) | |
| Fever | 0 | 1 (3%) | 0 | |
BMI: body mass index; CH4: methane; F: female; H2: hydrogen; M: male; OCTT: orocecal transit time; SIBO: small intestinal bacterial overgrowth.
A) SIBO eradication rate in the groups treated with rifaximin (n = 27), ciprofloxacin (n = 28), and metronidazole (n = 24). The data is graphed as number of patients; pink illustrates the percentage that managed to eradicate SIBO, and black represents those that maintained SIBO after treatment. B) H2 levels in parts per million (ppm), obtained by testing H2 and CH4 in expired air with lactulose, in subjects after antibiotic treatment. The values are expressed as the mean with standard error. Test with mixed effects adjustment model and Tukey post-test for multiple comparisons. *p value < 0.05. C) Comparison of H2 levels in ppm before and after antibiotic treatment with rifaximin. The values are expressed as mean with standard error. Test with two-way ANOVA and Bonferroni post-test for multiple comparisons. *p value < 0.05 D) Comparison of H2 levels in ppm before and after antibiotic treatment with ciprofloxacin. The values are expressed as mean with standard error. Test with mixed effects adjustment model and Tukey post-test for multiple comparisons. *p value < 0.05. E) Comparison of H2 levels in ppm before and after antibiotic treatment with metronidazole. The values are expressed as mean with standard error. Test with mixed effects adjustment model and Tukey post-test for multiple comparisons. *p value < 0.05. F) Comparison of H2 and CH4 levels in baseline ppm before and after antibiotic treatment with metronidazole. The values are expressed as median with interquartile range and analyzed with the Wilcoxon test. **p value < 0.01.
Regarding the post-treatment production of H2 and CH4, compared with the pre-antibiotic treatment breath test, rifaximin reduced H2 production at the 80-minute measurement (p value = 0.0492), with no differences at other times (Fig. 1C). Ciprofloxacin treatment significantly decreased H2 levels starting at 40 minutes (p value = 0.0143) (Fig. 1D). On the other hand, we observed a significant decrease in both H2 (Fig. 1E) and CH4 (Supplementary Fig. S1F) in subjects treated with metronidazole, both at the baseline (Fig. 1F) and at 20 (p value < 0.02) and 110 (p value < 0.05) minutes into the test, respectively, until the end of the test, suggesting an effective reduction of both SIBO and the colonic microbiota producing the two gases. We observed no significant differences in pre- and post-treatment CH4 levels in the groups treated with rifaximin or ciprofloxacin (Supplementary Fig. S1D and E). Additionally, we determined the OCTT, before and after treatment, observing no differences between the groups and no effects due to antibiotic treatment (Table 1).
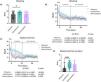
Symptomatic responseThere were no significant differences regarding symptoms at the beginning of the study, in terms of the intensity score of abdominal pain or the Bristol Stool Scale (Supplementary Fig. S2A and B). However, subjects in the ciprofloxacin group reported a higher intensity of bloating before antibiotic treatment (Fig. 2A). We used a linear regression model to assess symptomatology over time, in relation to each antibiotic, observing a significant decrease in the intensity of abdominal pain (Fig. 2B) and bloating (Fig. 2C) in all groups during treatment, reaching the minimum of abdominal pain on the fourth day of rifaximin treatment (Fig. 2D), compared with ciprofloxacin. Upon treatment completion, the score for each symptom remained low for 15 days prior to the control breath test, with no differences between the groups, regardless of SIBO eradication. There were no changes in terms of the frequency and consistency of bowel movements, according to the Bristol Stool Scale, during or after the treatments (Supplementary Fig. S2C and D).
A) Abdominal distension scale prior to treatment in subjects treated with rifaximin, ciprofloxacin, or metronidazole. The values are expressed as median with interquartile range and analyzed with the Kruskal-Wallis test and Dunn’s post-test. *p value < 0.05. B) Abdominal distention before, during, and after antibiotic treatment. The values are expressed as mean with standard error and a linear regression model was applied to evaluate the decrease in symptoms over time, in addition to comparing the slopes between treatments. C) Abdominal pain before, during, and after antibiotic treatment. The values are expressed as mean with standard error and a linear regression model was applied to evaluate the decrease in symptoms over time, in addition to comparing the slopes between treatments. Day 4 is highlighted in gray, where the greatest difference between treatments is observed. D) Comparison of abdominal pain scale on the fourth day after antibiotic treatment from Fig. 2C. The values are expressed as median with interquartile range and analyzed with the Kruskal-Wallis test and Dunn’s post-test ****p value <0.001.
Only 3 of the 32 subjects treated with rifaximin (9%) reported adverse effects due to treatment (Table 1); 2 of them presented with headache and one experienced vomiting. In contrast, 13 subjects treated with metronidazole and 13 treated with ciprofloxacin reported adverse effects (41% and 40%, respectively) (p value = 0.0026) that included nausea and headache, as well as others, to a lesser degree.
DiscussionThe patients with IBS and SIBO in our study showed significant symptom improvement, with the use of different antibiotics, and a reduced presence of SIBO based on H2. IBS patients often experience symptoms related to SIBO,16 but it can also be present in seemingly healthy individuals, ranging from 3 to 20%.10,12 The present study assessed the response to 3 antibiotics (rifaximin, ciprofloxacin, and metronidazole) in subjects with IBS and SIBO, aiming to enhance our understanding of the effectiveness of said treatments in a Chilean population. Our findings align with those of previous research, suggesting that SIBO is a significant comorbidity in patients with IBS. Our group has observed a high percentage of SIBO in patients with IBS, functional dyspepsia, and lactose intolerance, among other diseases.17–20
In our study, we demonstrated a high eradication percentage of SIBO, in 3 groups of patients treated with oral antibiotics. We also observed significant symptomatic improvement during treatment in all groups, particularly with respect to abdominal pain and bloating. This remained unchanged throughout the study, regardless of subsequent breath test results, leading us to believe other pathophysiologic mechanisms are involved, beyond SIBO. Rifaximin proved to be particularly effective in reducing symptoms after treatment, independent of SIBO eradication, which is consistent with previous studies highlighting its bacterial selectivity and safety profile.21–25
When comparing the efficacy of the 3 antibiotics, in terms of SIBO eradication, we found that metronidazole had superior efficacy, compared with rifaximin and ciprofloxacin, with these last two showing comparable responses. This finding could be attributed to differences in the spectrum of activity and tissue absorption of the antibiotics.26–28 A recently published systematic review29 demonstrated a higher symptomatic response to SIBO in patients with IBS, as our study also indicates. In a meta-analysis by Shah et al.,14 51% of patients treated with metronidazole had remission, assessed by a breath test. The remission rate after treatment with quinolones is more difficult to evaluate due to the limited number of studies. Ciprofloxacin is known to be effective against Gram-negative bacteria, whereas metronidazole is effective against anaerobic or microaerophilic microorganisms. Another randomized study showed that breath test normalization occurred more frequently after rifaximin treatment, compared with the use of metronidazole (63.4% vs 43.7%).30 Another aspect to consider is treatment cost in Latin America, motivating us to seek alternatives to rifaximin. In Chile, the average cost of a 14-day course of metronidazole is 4.80 USD, compared with the significantly higher 70.9 USD for rifaximin.
Among the limitations of our study, not using a placebo group could have led to an overestimation of treatment-related symptom improvement. Although patients were randomized without knowing which treatment they received, they were aware that they would receive antibiotics for the study. Although in routine clinical practice in Chile we use between 400 mg BID or TID, the national public health institute has only approved rifaximin at doses of 200 mg every 8 hours or 600 mg per day, for the treatment of traveler’s diarrhea but not for SIBO. Therefore, for the present intervention, our ethics committee accepted a maximum of 800 mg per day or 400 mg every 12 hours, without exceeding this dose, which resembles the 550 mg every 12 hours recommended internationally. Favorably, the efficacy of this regimen proved to be similar to that described in studies with doses of 550 mg BID. This dosage has also been tested in the Asian population with good response.
On the other hand, the limited duration of follow-up (15 days) may not be sufficient to assess sustained SIBO eradication or future symptom reappearance. Therefore, larger and longer-term studies conducted on our population are needed to assess the durability of the response to the antibiotics we analyzed and to evaluate possible therapeutic combinations. Our findings showed the effectiveness of rifaximin, reflected in reduced hydrogen levels, but not methane levels, unlike metronidazole, which decreased the levels of both gases. Therefore, future research on the profile of therapy-resistant gut microbiota could contribute to optimizing the use of rifaximin in this patient population. In summary, our study highlights the effectiveness of rifaximin, ciprofloxacin, and metronidazole in improving IBS symptoms in patients with SIBO. Our results support the consideration of rifaximin as a preferred treatment option in patients with IBS and SIBO, given its efficacy and safety profile. However, the choice of antibiotic should be based on individual patient assessment and the analysis of possible side effects and bacterial resistance. These findings collectively support the need to consider antibiotic treatment in the comprehensive management of patients with IBS. Nevertheless, further research is required to improve our understanding of said treatments and optimize therapeutic strategies in cases of recurrence.
CRediT authorship contribution statementCVM: Drafting, review, and translation. GL: Patient recruitment and statistical analysis. AM: Original idea, review, patient recruitment.
Declaration of Generative AI and AI-assisted technologies in the writing processNo AI tools were utilized.
Financial disclosureThe present study was funded by the gastroenterology section of the Clinical Hospital of the University of Chile, through internal research funds.
The following are Supplementary data to this article:
A–C) H2 and CH4 levels in parts per million (ppm), obtained upon measuring H2 and CH4 in exhaled air with lactulose in subjects before and after antibiotic treatment. The values are expressed as mean with standard deviation (SD). D–E) Comparison of the CH4 levels in ppm before and after antibiotic treatment with rifaximin, ciprofloxacin, or metronidazole. The values are expressed as mean with standard deviation. Test with mixed-effects model and Tukey post hoc test for multiple comparisons.
* p value < 0.05.