The diagnosis of minimal hepatic encephalopathy (MHE) is complex in clinical practice, given that its adequate evaluation is time-consuming. The animal naming test (ANT1) has proven to be a useful tool for rapid MHE identification. Our aim was to validate the ANT1 in a cohort of Mexican patients with cirrhosis of the liver.
Material and methodsAn observational, cross-sectional, and analytic study was conducted within the time frame of June 2022 and May 2023. MHE diagnosis was made using the psychometric hepatic encephalopathy score (PHES). Patients with overt HE evaluated through the West Haven criteria were excluded. The ANT1 was performed on all participants.
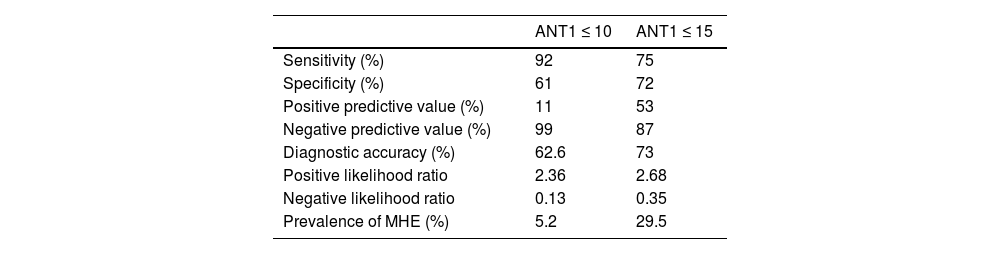
ResultsA total of 199 patients with cirrhosis and 31 non-cirrhotic control subjects were analyzed. Women predominated (61.8% and 71%) and mean patient age was 55 ± 10 and 53.6 ± 12 (range 19–84 years), respectively. Ninety patients (45.2%) met the MHE criteria determined by the PHES. Using an ANT1 cutoff point of ≤15, MHE was identified in 65 (32.7%) patients, along with 75% sensitivity, 72% specificity, 53% positive predictive value, 87% negative predictive value, and 73% diagnostic accuracy. The area under the curve for diagnosing MHE was 0.763 (standard error, 0.081; 95% confidence interval, 0.604−0.923; p ≤ 0.0001).
ConclusionsThe ANT1 was shown to be a useful tool for identifying MHE in daily clinical practice. In our population, a cutoff point ≤15 animals named could be utilized for rapid screening of patients at high risk for progressing to overt HE, who would then require extensive testing.
El diagnóstico de encefalopatía hepática mínima (EHM) es complejo en la práctica clínica ya que requiere invertir mucho tiempo para su adecuada evaluación. La prueba de denominación de animales (ANT1, siglas en inglés) ha demostrado ser una herramienta útil y rápida para la identificación de la EHM.
ObjetivoValidar la prueba ANT1 en una cohorte de pacientes mexicanos con cirrosis hepática.
Material y métodosEstudio observacional, transversal y analítico realizado durante el periodo junio de 2022 a mayo de 2023. El diagnóstico de EHM se realizó con el puntaje psicométrico de encefalopatía hepática (PHES, siglas en inglés), se excluyeron pacientes con EH manifiesta evaluada con criterios de West Haven. Se realizó prueba ANT1 en todos los participantes.
ResultadosSe analizaron 199 pacientes con cirrosis y 31sujetos en grupo control sin cirrosis, con predominio de mujeres (61.8% y 71%), edad 55 ± 10 y 53.6 ± 12 (rango 19 a 84 años). Noventa pacientes (45.2%) cumplieron criterios para EHM por PHES. Considerando ANT1 con punto de corte ≤15 se identificó EHM en 65 (32.7%) pacientes, así como sensibilidad de 75%, especificidad 72%, valor predictivo positivo 53%, valor predictivo negativo 87% y precisión diagnóstica 73%. El área bajo la curva para el diagnóstico de EHM fue 0.763 (error estándar de 0.081, intervalo de confianza del 95%, 0.604−0.923, p ≤ 0.0001).
ConclusionesLa prueba ANT1 demostró ser una herramienta útil para la identificación de EHM en la práctica clínica diaria. En nuestra población el punto de corte ≤15 animales podría ser utilizado para el escrutinio rápido de pacientes con alto riesgo de progresión a EH manifiesta que requiera someterse a pruebas exhaustivas.
Hepatic encephalopathy (HE) is a frequent complication of chronic liver disease. The risk for presenting with a first episode varies from 5 to 25% during the first five years from diagnosis, which leads to repercussions on survival and a high risk of recurrence, diminishing the quality of life of both patients and their caregivers. HE is defined as brain dysfunction caused by liver failure (acute or chronic) that manifests as neurologic or psychiatric alterations, ranging in severity from subclinical changes that are difficult to identify in the conventional medical examination to coma. Severity is evaluated through the West Haven clinical scale that divides it into covert HE and overt HE (grades II to IV).1
Covert HE encompasses minimal hepatic encephalopathy (MHE), identified only through specific tests and West Haven grade I, and is characterized by mild hypokinesia, psychomotor slowing, and inattention.2
The prevalence of MHE or covert HE is high, accounting for 20–80% of patients with cirrhosis, with a risk of progressing to overt HE. Because MHE presents with no clinical or cognitive signs in the conventional medical examination, psychometric and neurophysiologic evaluation strategies must be implemented. The diagnostic gold standard is the psychometric hepatic encephalopathy score (PHES), which consists of five paper-and-pencil tests that evaluate visuomotor coordination and psychomotor and cognitive processing speed.3 However, the test takes 15−20 min to be carried out, making its implementation in clinical daily practice difficult. Thus, different tools have been developed that facilitate an easier and faster evaluation, such as the critical flicker frequency (CFF) test, the continuous reaction time test, the inhibitory control test, the Stroop test, and recently, the animal naming test (ANT1).4–7
The ANT1 is a useful instrument of rapid application for the timely detection of MHE. It has not been studied in the Mexican population, and so our aim was to validate the ANT1 in a cohort of Mexican patients with hepatic cirrhosis.
Material and methodsStudy design and participantsAn observational, analytic, cross-sectional, multicenter study was conducted on patients from the Instituto de Investigaciones Médico-Biológicas of the Universidad Veracruzana, the Hospital Civil Fray Antonio Alcalde in Guadalajara, and the Hospital General de México “Dr. Eduardo Liceaga” in Mexico City, within the time frame of June 2022 and May 2023.
PatientsPatients that had cirrhosis of the liver diagnosed through imaging studies (ultrasound or transient liver elastography), endoscopic data, a complete medical history, and that were above 18 years of age were included in the study. Subjects under 18 years of age, with overt HE clinically evaluated through the West Haven criteria, patients undergoing management with drugs to reduce ammonia levels at the time of the evaluation and for the previous six weeks, those with alcohol or psychotropic drug use in the past three months, a history of transjugular intrahepatic portosystemic shunt placement, active infection, a history of cerebral vascular disease, electrolyte imbalance (serum potassium <3.5 mg/dl or >5 mg/dl, serum sodium <130 mg/dl or >150 mg/dl), chronic kidney disease, or active cancer at any stage were excluded. Subjects with an incomplete clinical file or duplicate registers were eliminated from the study.
The control group was made up of individuals above 18 years of age, in whom hepatic cirrhosis was ruled out through transient liver elastography (FibroScan®, Echosens, Paris, France).
Clinical evaluationTotal bilirubin, transaminase, albumin, hemoglobin, platelet, sodium, potassium, and creatinine levels and the international normalized ratio (INR) were evaluated in all the patients. The model for end-stage liver disease-sodium (MELD-Na) score and Child-Pugh classification were calculated to determine liver disease severity. The control group included patients diagnosed with fatty liver with no signs of fibrosis (fewer than 6.4 kPa), evaluated through FibroScan® transient liver elastography.
Hepatic encephalopathy evaluationThe presence of HE was clinically evaluated using the West Haven criteria, according to the recommendations of the joint guideline published by the American Association for the Study of Liver Diseases/European Association for the Study of the Liver (AASLD/EASL). If no clinical signs of overt HE were found, the PHES and ANT1 were carried out.
PHES: This test includes the number connection tests (NCT-A and NCT-B), digit symbol test, serial dotting test, and line tracing test for time (t) and error (e). A score ≤ −4 was considered diagnostic for MHE.8
ANT1: This test evaluates semantic fluency and consists of having the patient name as many animals as possible, for one minute. Help or clues were given if the patient could not name any animal within the first 15 seconds or if he/she stopped before the minute was up. At the end of the time limit, repetitions and errors were eliminated. Adjustments by age and educational level were made by adding three animals for patients with an educational level of fewer than eight years and age above 80 years. A score ≤15 was considered diagnostic of MHE.9 All tests were performed on the same day by trained physicians and in a quiet area with good lighting.
Statistical analysisThe numerical variables were reported as measures of central tendency and dispersion, and the categorical variables as frequencies and percentages. Data distribution was evaluated through the Kolmogorov-Smirnov test, and homoscedasticity through the Levene’s test. Means were compared utilizing the Student’s t test or the Wilcoxon rank-sum test, as appropriate. The categorical variables were compared through the chi-square test. The analysis of the ANT1, for distinguishing patients with MHE from those without it, was performed with an area under the receiver operating characteristic (AUROC) curve, with a 95% confidence interval (CI). The cutoff point maximizing the Youden index was determined, and the cutoff point offering the best sensitivity and specificity performance was calculated. In addition, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated with the cutoff points established by Campagna, et al.9 Statistical significance was set at a p < 0.05 and the result analysis was carried out using the IBM SPSS version 26 program (Chicago, Ill, USA).
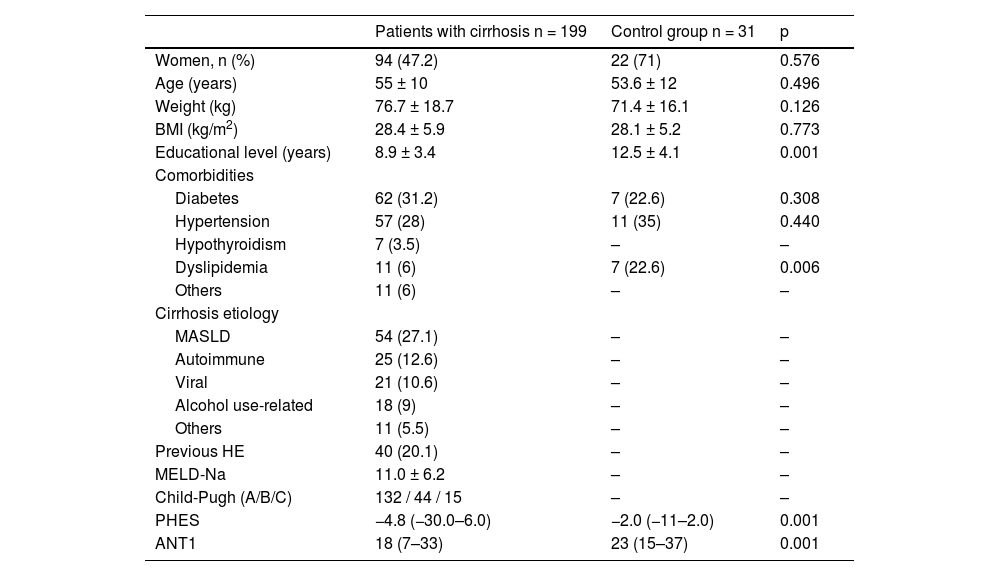
ResultsPopulation characteristicsA total of 199 patients with cirrhosis of the liver, who met the inclusion criteria, were included in the study. The majority of the patients were women (61.8%) and mean patient age was 55 ± 10 years (range: 19–84 years). The most frequent etiology was metabolic dysfunction-associated steatotic liver disease (27.1%), followed by autoimmune disease (12.6%), viral disease (10.6%), and disease related to alcohol use (9%). Mean educational level in years was 8.9 ± 3.4 (range: 0–20 years). Regarding liver disease severity, the mean MELD-Na score was 11.0 ± 6.3 and the majority of patients were classified as Child-Pugh class A (66.3%). The majority of patients had presented with one episode of decompensation: 84 (42.2%) had a history of gastrointestinal bleeding, 45 (22.6%) a history of ascites, and 40 (20.1%) a history of previous HE. The control group included 31 subjects with no liver fibrosis, determined through transient liver elastography with FibroScan® (fewer than 6.4 kPa); there was a predominance of women (71%), mean age of the controls was 53.6 ± 12 years, and body mass index (BMI) and comorbidities were similar to those of the group with cirrhosis. Table 1 describes the rest of the characteristics and Table 2 shows the results of the PHES and ANT1 for the control group and the patients with cirrhosis.
Characteristics of the patients with cirrhosis and the control group.
| Patients with cirrhosis n = 199 | Control group n = 31 | p | |
|---|---|---|---|
| Women, n (%) | 94 (47.2) | 22 (71) | 0.576 |
| Age (years) | 55 ± 10 | 53.6 ± 12 | 0.496 |
| Weight (kg) | 76.7 ± 18.7 | 71.4 ± 16.1 | 0.126 |
| BMI (kg/m2) | 28.4 ± 5.9 | 28.1 ± 5.2 | 0.773 |
| Educational level (years) | 8.9 ± 3.4 | 12.5 ± 4.1 | 0.001 |
| Comorbidities | |||
| Diabetes | 62 (31.2) | 7 (22.6) | 0.308 |
| Hypertension | 57 (28) | 11 (35) | 0.440 |
| Hypothyroidism | 7 (3.5) | – | – |
| Dyslipidemia | 11 (6) | 7 (22.6) | 0.006 |
| Others | 11 (6) | – | – |
| Cirrhosis etiology | |||
| MASLD | 54 (27.1) | – | – |
| Autoimmune | 25 (12.6) | – | – |
| Viral | 21 (10.6) | – | – |
| Alcohol use-related | 18 (9) | – | – |
| Others | 11 (5.5) | – | – |
| Previous HE | 40 (20.1) | – | – |
| MELD-Na | 11.0 ± 6.2 | – | – |
| Child-Pugh (A/B/C) | 132 / 44 / 15 | – | – |
| PHES | −4.8 (−30.0–6.0) | −2.0 (−11–2.0) | 0.001 |
| ANT1 | 18 (7–33) | 23 (15–37) | 0.001 |
ANT1: animal naming test; BMI: body mass index; HE: hepatic encephalopathy; MASLD: metabolic dysfunction-associated steatotic liver disease; MELD-Na; model for end-stage liver disease-sodium; PHES: psychometric hepatic encephalopathy score.
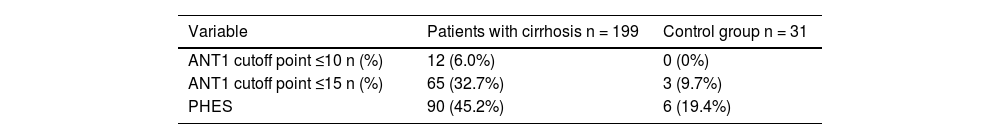
PHES and ANT1 results of the patients with cirrhosis and the control group.
| Variable | Patients with cirrhosis n = 199 | Control group n = 31 |
|---|---|---|
| ANT1 cutoff point ≤10 n (%) | 12 (6.0%) | 0 (0%) |
| ANT1 cutoff point ≤15 n (%) | 65 (32.7%) | 3 (9.7%) |
| PHES | 90 (45.2%) | 6 (19.4%) |
ANT1: animal naming test; PHES: psychometric hepatic encephalopathy score.
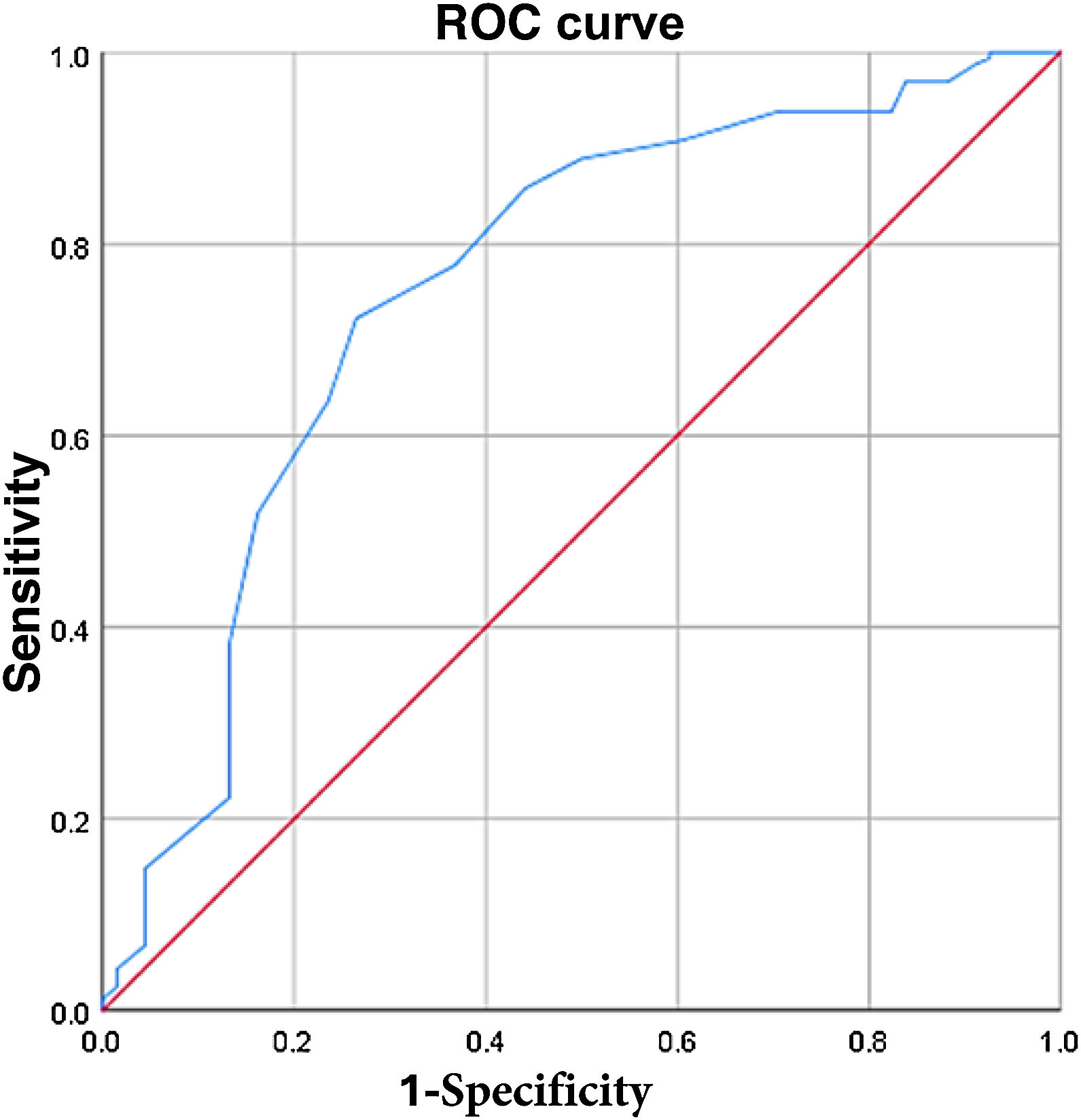
MHE was evaluated using the PHES, and 90 patients with cirrhosis of the liver (45.2%) had a positive result. Campagna et al.9 recommend cutoff points of ≤15 and ≤10 for identifying mild or serious cognitive decline. The ANT1 was considered positive with a cutoff point ≤15; MHE was identified in 65 (32.7%) patients and 134 (67.3%) patients had no signs of MHE, with 75% sensitivity, 72% specificity, 53% PPV, 87% NPV, and 73% diagnostic accuracy. The area under the curve for MHE diagnosis was 0.763 (standard error 0.081, 95% CI 0.604−0.923, p ≤ 0.0001) (Fig. 1). Patients with evidence of MHE through the PHES had a lower score on the ANT1, compared with the cirrhotic patients with no MHE and the controls (16.1 ± 5.1 versus 18 ± 5 and 23.6 ± 5.9, respectively, p ≤ 0.0001). The ANT1 scores correlated inversely with the MELD-Na scores (r = −0.249, p ≤ 0.0001) or Child-Pugh classifications (r = −0.258, p ≤ 0.0001) (Fig. 2).
ROC curve for the ANT1 ≤ 15 in patients with hepatic cirrhosis, with and without minimal hepatic encephalopathy. The ROC curve shows the sensitivity (vertical axis) compared with the 1-specificity (horizontal axis) of the ANT1 with a cutoff point ≤ 15 in differentiating patients with hepatic cirrhosis that present with minimal hepatic encephalopathy from those that do not.
ANT1: animal naming test. ROC: receiver operating characteristic.
With a cutoff point of ≤10 animals named, the ANT1 was positive in 12 patients (6.0%) with MHE and in none of the patients with no MHE data through the PHES, with 92% sensitivity, 61% specificity, 11% PPV, 99% NPV, and 62.6% diagnostic accuracy. The area under the curve for diagnosing MHE was 0.754 (standard error 0.038, 95% CI 0.680−0.828, p ≤ 0.0001) (Table 3).
Diagnostic quality of the ANT1 for detecting MHE, considering cutoff points of ≤10 and ≤15.
| ANT1 ≤ 10 | ANT1 ≤ 15 | |
|---|---|---|
| Sensitivity (%) | 92 | 75 |
| Specificity (%) | 61 | 72 |
| Positive predictive value (%) | 11 | 53 |
| Negative predictive value (%) | 99 | 87 |
| Diagnostic accuracy (%) | 62.6 | 73 |
| Positive likelihood ratio | 2.36 | 2.68 |
| Negative likelihood ratio | 0.13 | 0.35 |
| Prevalence of MHE (%) | 5.2 | 29.5 |
ANT1: animal naming test; MHE: minimal hepatic encephalopathy.
A sub-analysis was carried out, excluding patients above 75 years of age (3.5%, 7 patients). Considering the ANT1 positive, with a cutoff point ≤15, MHE was identified in 62 (32.3%) patients and 130 (67.7%) had no signs of MHE, with 55% sensitivity, 87% specificity, 77% PPV, 70% NPV, and 73% diagnostic accuracy. The area under the curve for diagnosing MHE was 0.709 (standard error 0.039, 95% CI 0.633−0.785, p ≤ 0.0001).
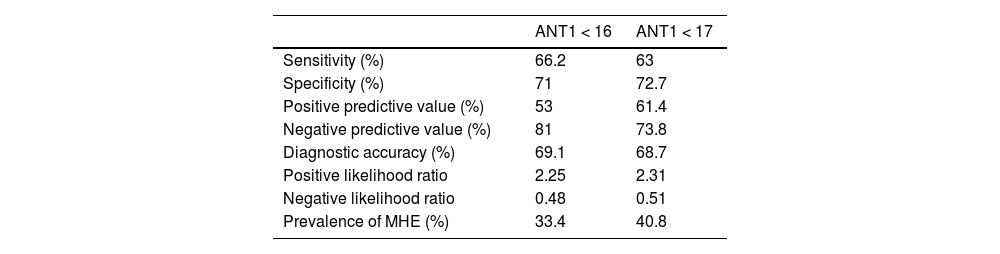
Evaluation of the cutoff points modified for the animal naming testThe diagnostic yield of the ANT1 was analyzed with other cutoff points in our population. Utilizing the cutoff point of ≤19 animals named, sensitivity was 57%, specificity was 41%, PPV was 60%, NPV was 38%, positive and negative likelihood ratios were 0.96 and 1.05, respectively, and the area under the curve was 0.739 (standard error 0.033, 95% CI 0.675−0.803, p ≤ 0.0001). We also analyzed the ANT1 with the cutoff points of 16 and 17 animals named. The cutoff point of ≤16 showed 66.2% sensitivity, 71% specificity, 53% PPV, 81% NPV, and 69.1% diagnostic accuracy. The area under the curve was 0.711 (standard error 0.037, 95% CI 0.638−0.784, p ≤ 0.0001). The cutoff point of ≤17 showed 63% sensitivity, 72.7% specificity, 61.4% PPV, 73.8% NPV, and 68.7% diagnostic accuracy. The area under the curve was 0.714 (standard error 0.035, 95% CI 0.645−0.782, p ≤ 0.0001). The prevalence of MHE was 33.4% and 40.8%, respectively (Table 4).
Diagnostic quality of the ANT1 for detecting MHE, considering cutoff values of <16 and <17.
| ANT1 < 16 | ANT1 < 17 | |
|---|---|---|
| Sensitivity (%) | 66.2 | 63 |
| Specificity (%) | 71 | 72.7 |
| Positive predictive value (%) | 53 | 61.4 |
| Negative predictive value (%) | 81 | 73.8 |
| Diagnostic accuracy (%) | 69.1 | 68.7 |
| Positive likelihood ratio | 2.25 | 2.31 |
| Negative likelihood ratio | 0.48 | 0.51 |
| Prevalence of MHE (%) | 33.4 | 40.8 |
ANT1: animal naming test; MHE: minimal hepatic encephalopathy.
Early identification of the minimal changes of HE is vitally important because they can cause sleep disorders, the impossibility to drive, risk for falls, and progression to overt HE, deteriorating the prognosis and quality of life of those patients.10 The prevalence of MHE in our study population was 45.2%, determined by the PHES. When different cutoff points for the ANT1 were considered, prevalence varied, but nevertheless, it was shown to be a useful tool because of its rapid identification and high level of sensitivity, even in the sub-analysis that excluded patients above 75 years of age.
There are numerous tools for diagnosing MHE but none of them are easily and quickly applied in daily clinical practice. The PHES questionnaires are recommended by the AASLD/EASL as the gold standard for diagnosing MHE.1–2 However, they require at least 15−20 min for application and interpretation, limiting the implementation of the test as a routine evaluation tool. Other methods, such as the inhibitory control test, the Stroop EncephalApp, and the CFF test, are useful for diagnosing MHE, but their application requires specialized equipment, is time-consuming, and requires trained personnel. Furthermore, they are not available at all centers due to their elevated cost. The limitations of those tests have restricted their routine application, which is essential for preventing progression to overt HE.11–13
Campagna et al. showed that the ANT1 is a practical and reliable tool for identifying MHE, with cutoff points of ≤15 and ≤10, which was validated in our study population, given that it was capable of discriminating patients with MHE from healthy controls. Depending on the cutoff point utilized, sensitivity and specificity could increase or decrease. In our study, a cutoff point of ≤15 resulted in better performance, with 75% sensitivity, 72% specificity, 53% PPV, 87% NPV, and 73% diagnostic accuracy. Said performance was similar to that described by Campagna et al., who reported 78% sensitivity, 63% specificity, 61% PPV, and 79% NPV.9
Reports have stated that the level of agreement between the different diagnostic tests for MHE can be low. Differences between populations and the low level of agreement between the diagnostic tests lead to the necessity of validating the cutoff point that is ideal for each population. Thus, we sought a cutoff point that would provide us with greater sensitivity for using the ANT1 as a tool for the timely detection of MHE, as well as for excluding a large number of patients with no alterations, who would not require specialized tests.14 The cutoff point of ≤15 animals named showed that 29.5% of the patients could be excluded from an extensive evaluation, with a 25% false negative rate. Agarwal et al. carried out a similar analysis but with changes in the educational level and a cutoff point of ≤14 animals named. They reported 89% sensitivity and 95% specificity, resulting in the proposal that there should not be one generalized cutoff point for the ANT1, but rather cutoff points should be adapted to different populations.5
Our study has weaknesses. The exclusion of patients with electrolyte imbalance, a history of transjugular intrahepatic portosystemic shunt placement, and chronic kidney disease, among other comorbidities, limits generalizing the results to all patients with cirrhosis of the liver. No episodes of previous overt HE were analyzed, excluding only those patients that reported episodes in the six weeks prior to the evaluation. Although patients with neurologic alterations were excluded, we cannot guarantee that other factors, such as mild cognitive decline (specific tests such as the mini-mental test or the Montreal cognitive assessment [MoCA] were not performed), traumatic brain injury, or small vessel disease, did not interfere in the interpretation of the PHES and ANT1. Comparison with the control group was included but the number of controls was small, and their educational levels were different, which could influence the test results. We believe that, even though the ANT1 is a useful tool, it is not specific for diagnosing MHE, but rather enables rapid screening to identify patients at high-risk of overt HE that require a more extensive diagnostic approach.
Currently, there is an insufficient number of studies conducted on the ANT1 in Mexican patients with cirrhosis of the liver. Therefore, more information on larger sample sizes is needed to verify the reliability of the test, as well as long-term follow-up to evaluate its predictive capacity.
ConclusionThe ANT1 proved to be a useful tool for identifying MHE in our study population. We found that the cutoff point of ≤15 animals named could be useful for the rapid screening of Mexican patients at high risk for progression to overt HE, who need to undergo extensive testing.
Ethical considerationsTo guarantee meeting and adhering to ethics and research norms, all the study participants gave their informed consent prior to the evaluations. The study was reviewed and approved by the Research and Ethics Committee of our institutions (llMB-2020-09), and strictly followed the official data protection regulations and ethical principles established by the Declaration of Helsinki. The data of the participants were treated with the utmost confidentiality and security measures, including de-identification and access restricted to authorized personnel. The participants could leave the study at any time, with no penalization measures taken.
Financial disclosureNo financial support was received in relation to this article.